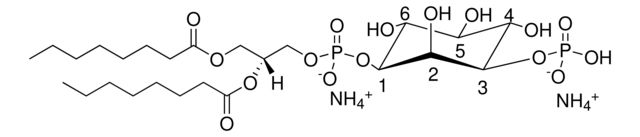
850185P
08:0 PI(4,5)P2
Manufacturer: Sigma Aldrich
CAS Number: 852043-37-9
Synonym(S): PIP2[4′,5′](8:0/8:0); 110665
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 100 μG | 850185P-100-μG | In Stock | ₹ 12,243.08 |
| 500 μG | 850185P-500-μG | In Stock | ₹ 48,939.83 |
850185P - 100 μG
In Stock
Quantity
1
Base Price: ₹ 12,243.08
GST (18%): ₹ 2,203.754
Total Price: ₹ 14,446.834
description
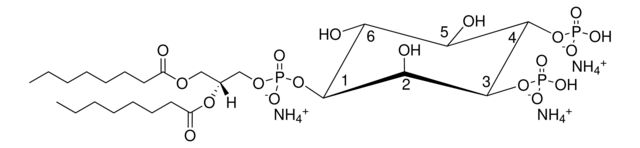
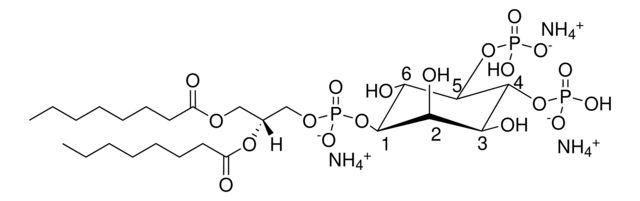
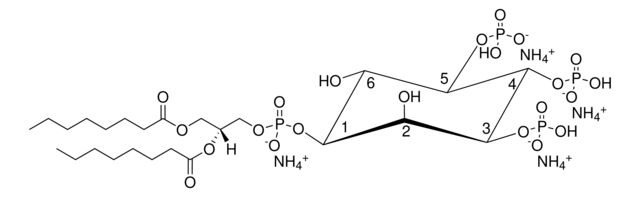
1,2-dioctanoyl-sn-glycero-3-phospho-(1′-myo-inositol-4′,5′-bisphosphate) (ammonium salt)
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 100 μg (with stopper and crimp cap (850185P-100ug))pkg of 1 × 500 μg (with stopper and crimp cap (850185P-500ug))
manufacturer/tradename
Avanti Polar Lipids
lipid type
cardiolipinsphospholipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
[H][C@@](COP([O-])(O[C@H]1[C@H](O)[C@@H](OP(O)([O-])=O)[C@H](OP([O-])(O)=O)[C@@H](O)[C@H]1O)=O)(OC(CCCCCCC)=O)COC(CCCCCCC)=O.[NH4+].[NH4+].[NH4+]
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | 852043-37-9 | 1,2-dioctanoyl-sn-glycero-3-phospho-(1'-myo-inositol-4',5'-bisphosphate) (ammonium salt) | A2B Chem | -- |
Related Products
Description
- General description: Although PI(4,5)P2 is a minor component of cell membranes, it plays a critical role as a substrate for a number of important signaling proteins. PI(4,5)P2 is an intermediate in the IP3/DAG pathway where it is hydrolyzed by phospholipase C to liberate the second messengers, inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). PI(4,5)P2 is also a substrate for PI 3-kinase where it is phosphorylated to PI(3,4,5)P3, an activator of downstream signaling components such as the protein kinase AKT. The dioctanoyl derivative of PI(4,5)P2 is more water soluble than its long chain counterparts.
- Application: 08:0 PI(4,5)P2 is suitable for use to identify its binding site in transient receptor potential vanilloid 4 (TRPV4 N) terminus.[1] It has been used in in vitro binding assay to determine the binding of G-protein coupled receptor kinases (GRKs) and β2-adrenergic receptor (β2AR).[2]
- Packaging: 2 mL Amber Serum Vial with Stopper and Crimp Cap (850185P-100ug)
Compare Similar Items
Show Difference
description: 1,2-dioctanoyl-sn-glycero-3-phospho-(1′-myo-inositol-4′,5′-bisphosphate) (ammonium salt)
Assay: >99% (TLC)
form: powder
packaging: pkg of 1 × 100 μg (with stopper and crimp cap (850185P-100ug))pkg of 1 × 500 μg (with stopper and crimp cap (850185P-500ug))
manufacturer/tradename: Avanti Polar Lipids
lipid type: cardiolipinsphospholipids
shipped in: dry ice
storage temp.: −20°C
SMILES string: [H][C@@](COP([O-])(O[C@H]1[C@H](O)[C@@H](OP(O)([O-])=O)[C@H](OP([O-])(O)=O)[C@@H](O)[C@H]1O)=O)(OC(CCCCCCC)=O)COC(CCCCCCC)=O.[NH4+].[NH4+].[NH4+]
description:
1,2-dioctanoyl-sn-glycero-3-phospho-(1′-myo-inositol-4′,5′-bisphosphate) (ammonium salt)
Assay:
>99% (TLC)
form:
powder
packaging:
pkg of 1 × 100 μg (with stopper and crimp cap (850185P-100ug))pkg of 1 × 500 μg (with stopper and crimp cap (850185P-500ug))
manufacturer/tradename:
Avanti Polar Lipids
lipid type:
cardiolipinsphospholipids
shipped in:
dry ice
storage temp.:
−20°C
SMILES string:
[H][C@@](COP([O-])(O[C@H]1[C@H](O)[C@@H](OP(O)([O-])=O)[C@H](OP([O-])(O)=O)[C@@H](O)[C@H]1O)=O)(OC(CCCCCCC)=O)COC(CCCCCCC)=O.[NH4+].[NH4+].[NH4+]
description: __
Assay: >99% (TLC)
form: powder
packaging: pkg of 1 × 100 μg (with stopper and crimp cap (850186P-100ug))pkg of 1 × 500 μg (with stopper and crimp cap (850186P-500ug))
manufacturer/tradename: Avanti Polar Lipids 850186P
lipid type: phospholipidscardiolipins
shipped in: dry ice
storage temp.: −20°C
SMILES string: [H][C@@](COP(O[C@H]1[C@H](O)[C@@H](OP([O-])(O)=O)[C@H](OP(O)([O-])=O)[C@@H](OP(O)([O-])=O)[C@H]1O)([O-])=O)(OC(CCCCCCC)=O)COC(CCCCCCC)=O.[NH4+].[NH4+].[NH4+].[NH4+]
description:
__
Assay:
>99% (TLC)
form:
powder
packaging:
pkg of 1 × 100 μg (with stopper and crimp cap (850186P-100ug))pkg of 1 × 500 μg (with stopper and crimp cap (850186P-500ug))
manufacturer/tradename:
Avanti Polar Lipids 850186P
lipid type:
phospholipidscardiolipins
shipped in:
dry ice
storage temp.:
−20°C
SMILES string:
[H][C@@](COP(O[C@H]1[C@H](O)[C@@H](OP([O-])(O)=O)[C@H](OP(O)([O-])=O)[C@@H](OP(O)([O-])=O)[C@H]1O)([O-])=O)(OC(CCCCCCC)=O)COC(CCCCCCC)=O.[NH4+].[NH4+].[NH4+].[NH4+]
description: __
Assay: >99% (TLC)
form: powder
packaging: pkg of 1 × 100 μg (with stopper and crimp cap (850187P-100ug))pkg of 1 × 500 μg (with stopper and crimp cap (850187P-500ug))
manufacturer/tradename: Avanti Polar Lipids 850187P
lipid type: phospholipidscardiolipins
shipped in: dry ice
storage temp.: −20°C
SMILES string: [H][C@@](COP([O-])(O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](OP([O-])(O)=O)[C@H]1O)=O)(OC(CCCCCCC)=O)COC(CCCCCCC)=O.[NH4+].[NH4+]
description:
__
Assay:
>99% (TLC)
form:
powder
packaging:
pkg of 1 × 100 μg (with stopper and crimp cap (850187P-100ug))pkg of 1 × 500 μg (with stopper and crimp cap (850187P-500ug))
manufacturer/tradename:
Avanti Polar Lipids 850187P
lipid type:
phospholipidscardiolipins
shipped in:
dry ice
storage temp.:
−20°C
SMILES string:
[H][C@@](COP([O-])(O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](OP([O-])(O)=O)[C@H]1O)=O)(OC(CCCCCCC)=O)COC(CCCCCCC)=O.[NH4+].[NH4+]