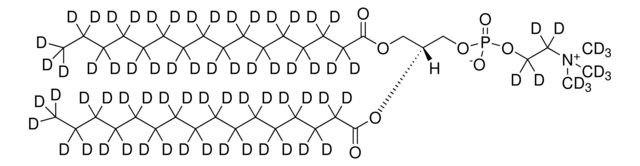
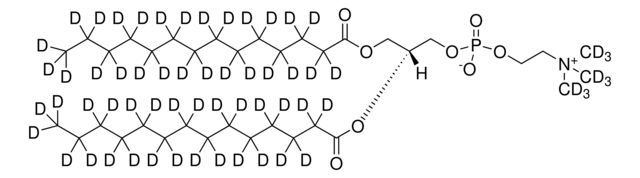
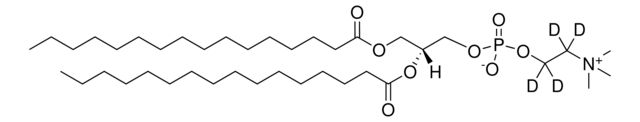
860347P
14:0 PC-d63
Manufacturer: Sigma Aldrich
CAS Number: 326495-30-1
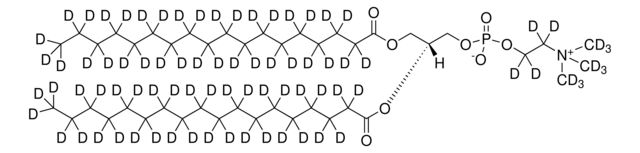
Synonym(S): 1,2-Dimyristoyl-d54-sn-glycero-3-phosphocholine-N,N,N-trimethyl-d9
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 10 MG | 860347P-10-MG | In Stock | ₹ 1,39,112.08 |
860347P - 10 MG
In Stock
Quantity
1
Base Price: ₹ 1,39,112.08
GST (18%): ₹ 25,040.174
Total Price: ₹ 1,64,152.254
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 10 mg (860347P-10MG)
manufacturer/tradename
Avanti Polar Lipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
[H][C@@](COP([O-])(OCC[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])=O)(OC(C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | 326495-30-1 | 1,2-dimyristoyl-d54-sn-glycero-3-phosphocholine-N,N,N-trimethyl-d9 | A2B Chem | ₹ 98,256.00 |
Related Products
Description
- General description: Phosphatidylcholine (PC) (Lecithins) has two fatty acid chains attached to a head group. Generally, PC has saturated fatty acid in the sn-1 position and unsaturated fatty acid in the sn-2 position. It has a quaternary ammonium group and a phosphate moiety in the head group, existing as a zwitterion in a wide range of pH. The fatty acid in PC is mostly palmitic, stearic, oleic, linolenic and arachidonic acid.[1] It is present abundantly in the mammalian membranes.[2] PC is principally synthesized from cytidine diphosphate-choline (CDP-choline).[1] Selective deuteration of this lipid may be used in the structural studies using techniques such as nuclear magnetic resonance (NMR), neutron reflectivity and small-angle neutron scattering.[3]
- Biochem/physiol Actions: Phosphatidylcholine (PC) is essential for cholesterol trafficking and membrane cholesterol homeostasis. It acts as the precursor for the synthesis of sphingomyelin.[4] PC plays a key role in the synthesis of second messenger and signaling molecules like diacyl glycerol, lysophosphatidic acid, phosphatidic acid and arachidonic acid.[5]
- Packaging: 5 mL Amber Glass Screw Cap Vial (860347P-10MG)
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
Assay: >99% (TLC)
form: powder
packaging: pkg of 1 × 10 mg (860347P-10MG)
manufacturer/tradename: Avanti Polar Lipids
shipped in: dry ice
storage temp.: −20°C
SMILES string: [H][C@@](COP([O-])(OCC[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])=O)(OC(C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2
Assay:
>99% (TLC)
form:
powder
packaging:
pkg of 1 × 10 mg (860347P-10MG)
manufacturer/tradename:
Avanti Polar Lipids
shipped in:
dry ice
storage temp.:
−20°C
SMILES string:
[H][C@@](COP([O-])(OCC[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])=O)(OC(C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2
Assay: >99% (TLC)
form: powder
packaging: pkg of 1 × 10 mg (860352P-10mg)
manufacturer/tradename: Avanti Polar Lipids 860352P
shipped in: dry ice
storage temp.: −20°C
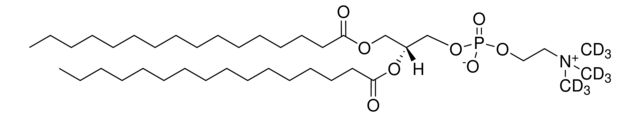
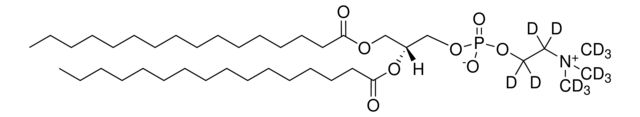
SMILES string: [H][C@@](COP([O-])(OCC[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])=O)(OC(CCCCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCCCC)=O
Assay:
>99% (TLC)
form:
powder
packaging:
pkg of 1 × 10 mg (860352P-10mg)
manufacturer/tradename:
Avanti Polar Lipids 860352P
shipped in:
dry ice
storage temp.:
−20°C
SMILES string:
[H][C@@](COP([O-])(OCC[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])=O)(OC(CCCCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCCCC)=O
Assay: >99% (TLC)
form: powder
packaging: pkg of 1 × 10 mg (860353P-10mg)
manufacturer/tradename: Avanti Polar Lipids 860353P
shipped in: dry ice
storage temp.: −20°C
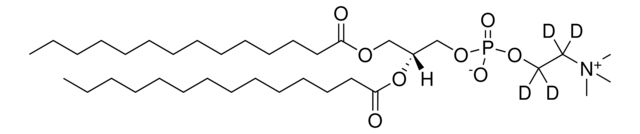
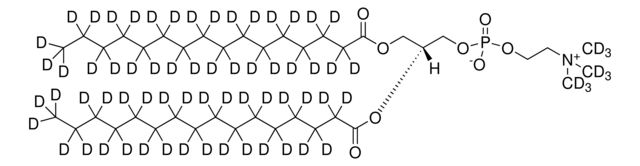
SMILES string: [H][C@@](COP([O-])(OC([2H])([2H])C([2H])([2H])[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])=O)(OC(CCCCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCCCC)=O
Assay:
>99% (TLC)
form:
powder
packaging:
pkg of 1 × 10 mg (860353P-10mg)
manufacturer/tradename:
Avanti Polar Lipids 860353P
shipped in:
dry ice
storage temp.:
−20°C
SMILES string:
[H][C@@](COP([O-])(OC([2H])([2H])C([2H])([2H])[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])=O)(OC(CCCCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCCCC)=O