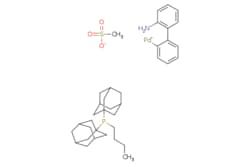
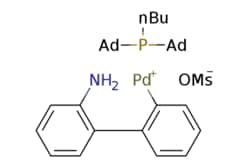
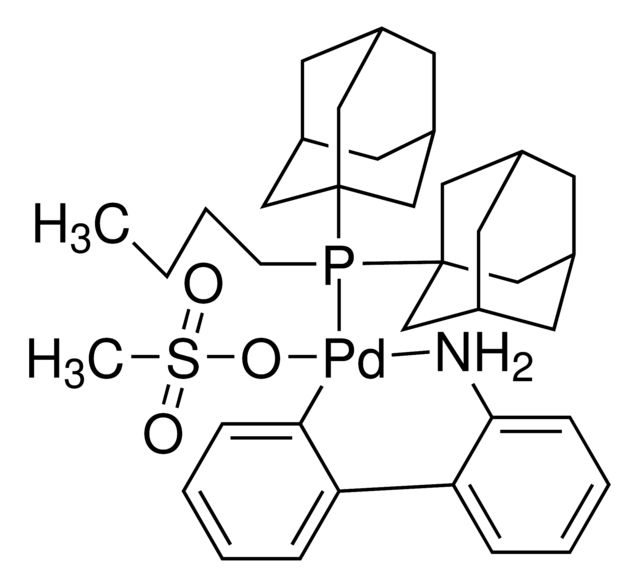
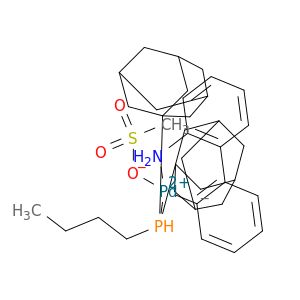
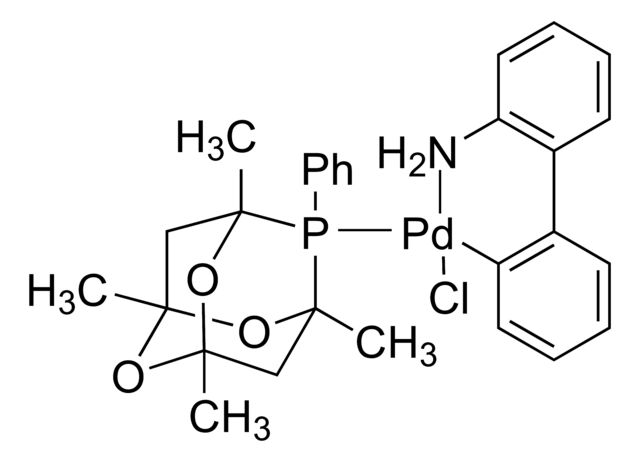
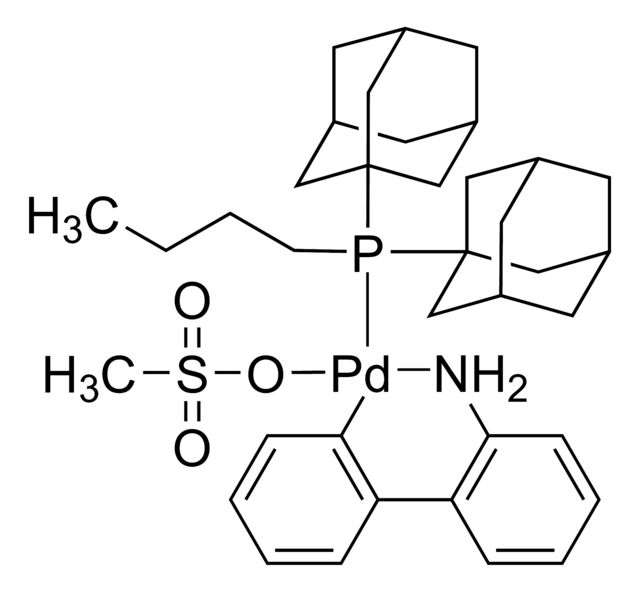
cataCXium® A Pd G3
95%
Manufacturer: Sigma Aldrich
CAS Number: 1651823-59-4
Synonym(S): Mesylate[(di(1-adamantyl)-n-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II), [(Di(1-adamantyl)-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate, cataCXium-A-Pd-G3
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| IND834-1G | 761435-IND834-1G | In Stock | ₹ 10,260.00 |
| 250 MG | 761435-250-MG | In Stock | ₹ 11,832.60 |
| 1 G | 761435-1-G | In Stock | ₹ 36,918.60 |
| IND834-5G | 761435-IND834-5G | In Stock | ₹ 48,320.00 |
| 5 G | 761435-5-G | In Stock | ₹ 1,36,851.90 |
761435 - IND834-1G
In Stock
Quantity
1
Base Price: ₹ 10,260.00
GST (18%): ₹ 1,846.80
Total Price: ₹ 12,106.80
Quality Level
100
Assay
95%
form
solid
feature
generation 3
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
impurities
≤3% acetone
mp
196-241 °C (decomposition)
functional group
phosphine
SMILES string
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CCCCP([C@@]34C[C@@H]5C[C@@H](C[C@@H](C5)C3)C4)[C@@]67C[C@@H]8C[C@@H](C[C@@H](C8)C6)C7
InChI
1S/C24H39P.C12H10N.CH4O3S.Pd/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h17-22H,2-16H2,1H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1/t17-,18+,19-,20-,21+,22-,23-,24-;;;
Other Options
Related Products
Description
- Application: cataCXium® A Pd G3 is a Buchwald third-generation precatalyst that can be used in:Direct ortho-arylation of pyridinecarboxylic acids.[1][2]Catalyzing Suzuki–Miyaura cross-coupling in the synthesis of 1-heteroaryl-3-azabicyclo[3.1.0]hexanes.[3]Palladium-catalyzed carbonylative carboperfluoroalkylation of alkynes.[4]Suzuki–Miyaura coupling reaction of geminal bis(boryl)cyclopropanes in the synthesis of various gem-disubstituted cyclopropanes.[5]Boroperfluoroalkylation of terminal alkynes.[6]Copper-free Sonogashira coupling reaction of aromatic halides with alkynes to form C-C bond.[7]Suzuki cross-coupling between organotrifluoroborate and aryl halides.[8]For small scale and high throughput uses, product is also available as ChemBeads (928305)
- Legal Information: cataCXium is a registered trademark of Umicore AG & Co. KG
SAFETY INFORMATION
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable