901220
EPhos Pd G4
Manufacturer: Sigma Aldrich
CAS Number: 2132978-44-8
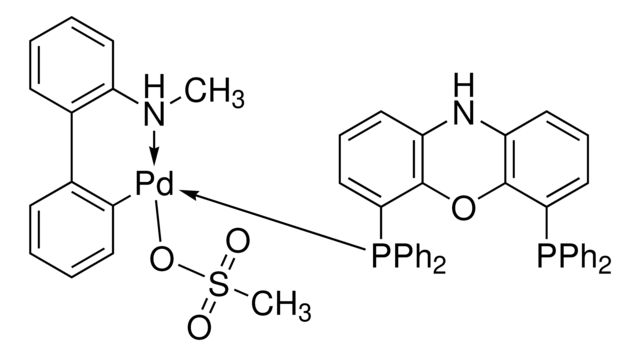
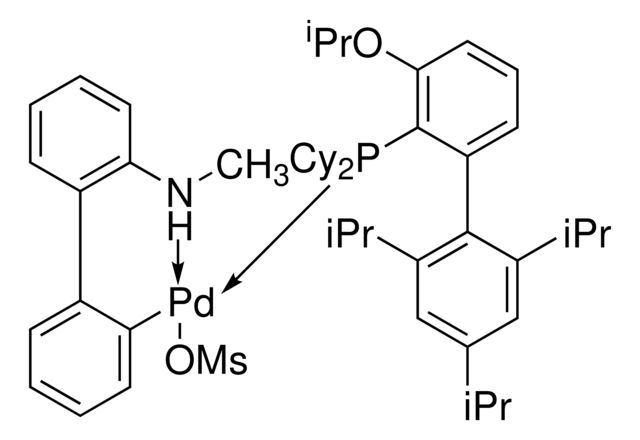
Synonym(S): [Dicyclohexyl[3-(1-methylethoxy)-2′,4′,6′-tris(1-methylethyl)[1,1′-biphenyl]-2-yl]phosphine-κP](methanesulfonato-κO)[2′-(methylamino-κN)[1,1′-biphenyl]-2-yl-κC]palladium
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 901220-1-G | In Stock | ₹ 35,919.60 |
901220 - 1 G
In Stock
Quantity
1
Base Price: ₹ 35,919.60
GST (18%): ₹ 6,465.528
Total Price: ₹ 42,385.128
form
powder or crystals
Quality Level
100
feature
generation 4
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
functional group
phosphine
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | EPhos Pd G4 | Aaron Chemicals LLC | ₹ 2,581.00 - ₹ 2,33,447.00 | |
 | 2132978-44-8 | EPhos Pd G4 | A2B Chem | ₹ 3,293.00 - ₹ 48,060.00 |
Related Products
Description
- Application: Buchwald palladacycle precatalyst with EPhos ligand for Pd-catalyzed C–N cross-coupling between primary amines and aryl halides to form 2-arylaminooxazoles. Catalyst system was reported with NaOPh (CDS001581).It is a fourth generation (G4) Buchwald precatalyst that is similar to the third generation (G3) precatalysts except that the amino group on the biphenyl backbone is methylated. This modification helps to prevent the limitations of using the third generation precatalysts. It is air, moisture and thermally-stable and shows good solubility in common organic solvents. BrettPhos Pd G4 is highly useful in cross-coupling reactions. Some of its excellent features include lower catalyst loadings, shorter reaction time, and efficient formation of the active catalytic species and accurate control of ligand: palladium ratio.
- Other Notes: Mechanistic Insight Leads to a Ligand That Facilitates the Pd-Catalyzed Formation of 2-(Hetero)Arylaminooxazoles and 4-(Hetero)Arylaminothiazoles
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
form: powder or crystals
Quality Level: 100
feature: generation 4
reaction suitability: reaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
functional group: phosphine
form:
powder or crystals
Quality Level:
100
feature:
generation 4
reaction suitability:
reaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
functional group:
phosphine