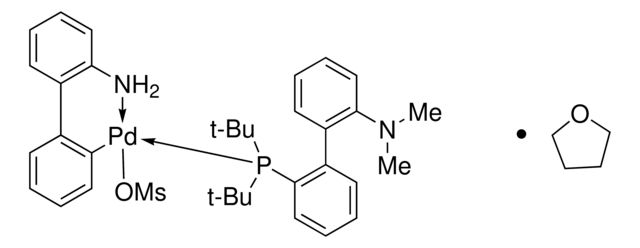
903701
tBuDavePhos Pd G3
1:1 THF adduct
Manufacturer: Sigma Aldrich
Synonym(S): t-BuDavePhos G3 Palladacycle, t-BuDavePhos Palladacycle
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 903701-1-G | In Stock | ₹ 16,720.00 |
903701 - 1 G
In Stock
Quantity
1
Base Price: ₹ 16,720.00
GST (18%): ₹ 3,009.60
Total Price: ₹ 19,729.60
form
powder or crystals
feature
generation 3
reaction suitability
core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
functional group
phosphine
SMILES string
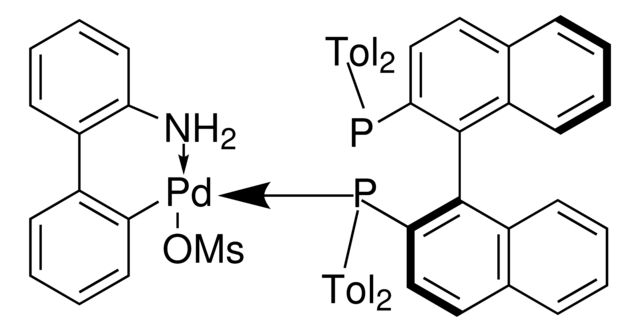
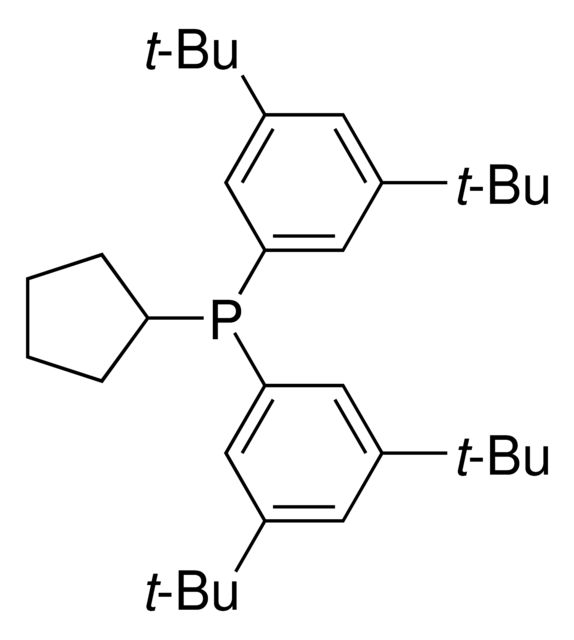
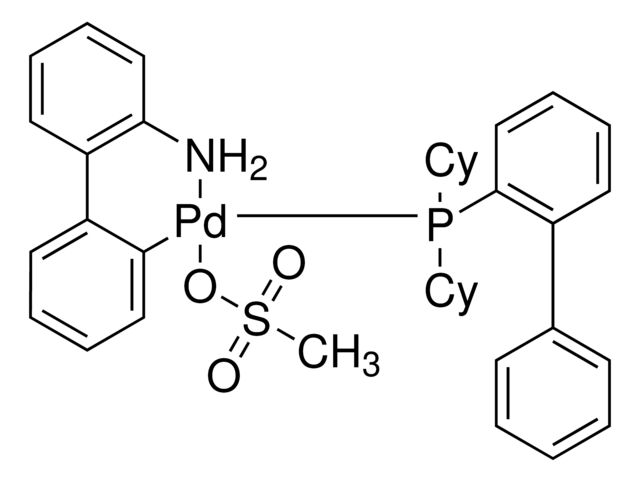
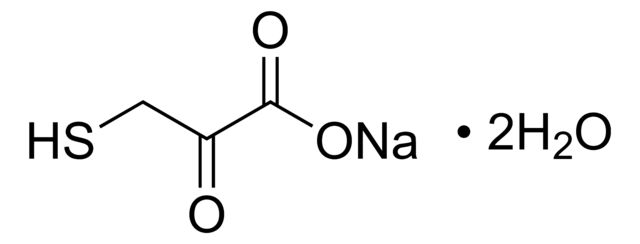
CN(C1=C(C2=C(P(C(C)(C)C)C(C)(C)C)C=CC=C2)C=CC=C1)C.NC3=C(C4=C([Pd]OS(C)(=O)=O)C=CC=C4)C=CC=C3.C5COCC5
Related Products
Description
- General description: tBuDavePhos Pd G3 is a third generation (G3) Buchwald precatalyst. It is air, moisture and thermally-stable and is highly soluble in a wide range of common organic solvents. It has a long life in solutions. tBuDavePhos Pd G3 is an excellent reagent for palladium catalyzed cross-coupling reactions. Some of its unique features include lower catalyst loadings, shorter reaction time, efficient formation of the active catalytic species and accurate control of ligand: palladium ratio.
- Application: tBuDavePhos Pd G3 has been used as a precatalyst for the Synthesis of Aryl Sulfonamides via Palladium-Catalyzed Chlorosulfonylation of Arylboronic Acids. It may also be used for a variety of C-C and C-N cross-coupling reactions.
- Other Notes: Technology Spotlight: G3 and G4 Buchwald PrecatalystsSynthesis of Aryl Sulfonamides via Palladium-Catalyzed Chlorosulfonylation of Arylboronic AcidsDesign and preparation of new palladium precatalysts for C-C and C-N cross-coupling reactions
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
form: powder or crystals
feature: generation 3
reaction suitability: core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
functional group: phosphine
SMILES string: CN(C1=C(C2=C(P(C(C)(C)C)C(C)(C)C)C=CC=C2)C=CC=C1)C.NC3=C(C4=C([Pd]OS(C)(=O)=O)C=CC=C4)C=CC=C3.C5COCC5
form:
powder or crystals
feature:
generation 3
reaction suitability:
core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
functional group:
phosphine
SMILES string:
CN(C1=C(C2=C(P(C(C)(C)C)C(C)(C)C)C=CC=C2)C=CC=C1)C.NC3=C(C4=C([Pd]OS(C)(=O)=O)C=CC=C4)C=CC=C3.C5COCC5