905607
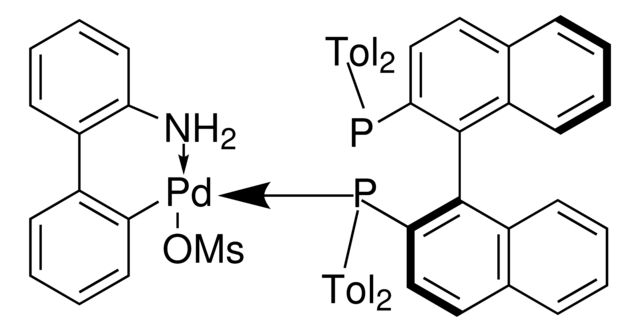
(R)-Tol-BINAP Pd G3
≥95%
Manufacturer: Sigma Aldrich
Synonym(S): (R)-Tol-BINAP G3 palladacycle, (R)-Tol-BINAP palladacycle
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 905607-1-G | In Stock | ₹ 30,158.70 |
905607 - 1 G
In Stock
Quantity
1
Base Price: ₹ 30,158.70
GST (18%): ₹ 5,428.566
Total Price: ₹ 35,587.266
Assay
≥95%
form
powder or crystals
feature
generation 3
reaction suitability
core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
mp
>300 °C
functional group
phosphine
SMILES string
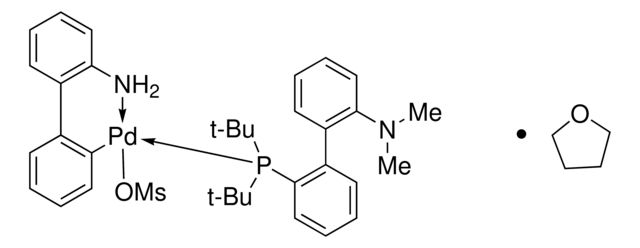
PC1=CC=C2C(C=CC=C2)=C1C3=C(C)C=CC4=C3C=CC=C4.NC(C=CC=C5)=C5C6=C([Pd]OS(C)(=O)=O)C=CC=C6.[Tol2].[Tol2].P
Related Products
Description
- General description: (R)-Tol-BINAP Pd G3 is a third generation (G3) Buchwald precatalyst. It is air, moisture and thermally stable and is highly soluble in a wide range of common organic solvents. It has long life in solutions. Qphos Pd G3 is an excellent reagent for palladium catalyzed cross-coupling reactions. Some of its unique features include lower catalyst loadings, shorter reaction time, efficient formation of the active catalytic species and accurate control of ligand: palladium ratio.
- Application: (R)-Tol-BINAP Pd G3 can be used in the stereoselective synthesis of perfluoroalkyl-substituted enones by reacting four components, alkynes, iodoperfluoroalkanes, (hetero)arylboronic acids, and carbon monoxide.[1]
- Other Notes: Pd-Catalyzed Carbonylative Carboperfluoroalkylation of Alkynes. Through-Space 13C–19F Coupling as a Probe for Configuration Assignment of Fluoroalkyl-Substituted Olefins
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
Assay: ≥95%
form: powder or crystals
feature: generation 3
reaction suitability: core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
mp: >300 °C
functional group: phosphine
SMILES string: PC1=CC=C2C(C=CC=C2)=C1C3=C(C)C=CC4=C3C=CC=C4.NC(C=CC=C5)=C5C6=C([Pd]OS(C)(=O)=O)C=CC=C6.[Tol2].[Tol2].P
Assay:
≥95%
form:
powder or crystals
feature:
generation 3
reaction suitability:
core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
mp:
>300 °C
functional group:
phosphine
SMILES string:
PC1=CC=C2C(C=CC=C2)=C1C3=C(C)C=CC4=C3C=CC=C4.NC(C=CC=C5)=C5C6=C([Pd]OS(C)(=O)=O)C=CC=C6.[Tol2].[Tol2].P
Assay: ≥95%
form: powder or crystals
feature: __
reaction suitability: core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalyst
mp: >300 °C
functional group: __
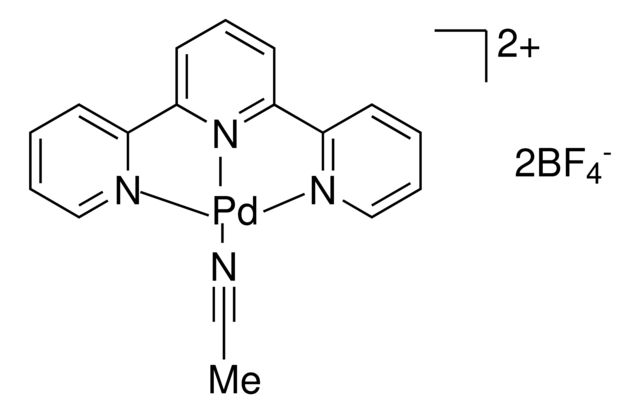
SMILES string: N#CC.C1(C2=CC=CC=N2)=CC=CC(C3=NC=CC=C3)=N1.[Pd]
Assay:
≥95%
form:
powder or crystals
feature:
__
reaction suitability:
core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalyst
mp:
>300 °C
functional group:
__
SMILES string:
N#CC.C1(C2=CC=CC=N2)=CC=CC(C3=NC=CC=C3)=N1.[Pd]
Assay: __
form: powder
feature: __
reaction suitability: core: iridiumreagent type: catalystreaction type: Photocatalysis
mp: __
functional group: __
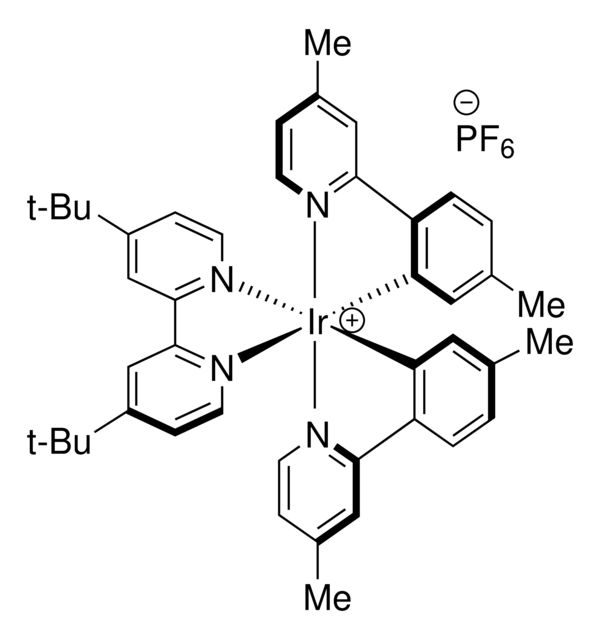
SMILES string: F[P-](F)(F)(F)(F)F.CC1=CC([Ir+]([N]2=C3C=C(C)C=C2)(C4=C5C=CC(C)=C4)([N]6=C7C=C(C(C)(C)C)C=C6)([N]8=C7C=C(C(C)(C)C)C=C8)[N]9=C5C=C(C)C=C9)=C3C=C1
Assay:
__
form:
powder
feature:
__
reaction suitability:
core: iridiumreagent type: catalystreaction type: Photocatalysis
mp:
__
functional group:
__
SMILES string:
F[P-](F)(F)(F)(F)F.CC1=CC([Ir+]([N]2=C3C=C(C)C=C2)(C4=C5C=CC(C)=C4)([N]6=C7C=C(C(C)(C)C)C=C6)([N]8=C7C=C(C(C)(C)C)C=C8)[N]9=C5C=C(C)C=C9)=C3C=C1