760676
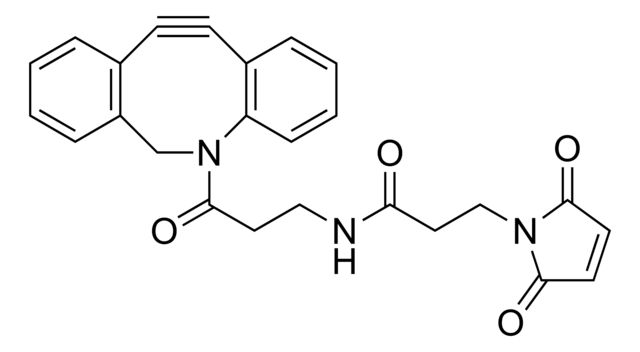
Dibenzocyclooctyne-PEG4-maleimide
for Copper-free Click Chemistry
Manufacturer: Sigma Aldrich
Synonym(S): Polyethylene glycol, DBCO-PEG4-maleimide
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 MG | 760676-1-MG | In Stock | ₹ 2,662.95 |
| 5 MG | 760676-5-MG | In Stock | ₹ 8,595.05 |
| 50 MG | 760676-50-MG | In Stock | ₹ 32,810.58 |
760676 - 1 MG
In Stock
Quantity
1
Base Price: ₹ 2,662.95
GST (18%): ₹ 479.331
Total Price: ₹ 3,142.281
form
solid
reaction suitability
reaction type: click chemistryreagent type: cross-linking reagent
functional group
maleimide
storage temp.
−20°C
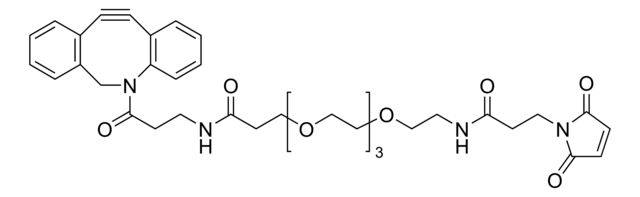
SMILES string
O=C(CCNC(CCOCCOCCOCCOCCNC(CCN1C(C=CC1=O)=O)=O)=O)N2CC3=C(C=CC=C3)C#CC4=C2C=CC=C4
InChI
1S/C36H42N4O9/c41-32(14-18-39-34(43)11-12-35(39)44)38-17-20-47-22-24-49-26-25-48-23-21-46-19-15-33(42)37-16-13-36(45)40-27-30-7-2-1-5-28(30)9-10-29-6-3-4-8-31(29)40/h1-8,11-12H,13-27H2,(H,37,42)(H,38,41)
InChI key
VVFZXPZWVJMYPX-UHFFFAOYSA-N
Related Products
Description
- Application: Maleimide functionalized cyclooctyne derivative for incorporation of the cyclooctyne moiety into thiol containing compounds or biomolecules. Cyclooctynes are useful in strain-promoted copper-free azide-alkyne cycloaddition reactions. This dibenzocyclooctyne will react with azide functionalized compounds or biomolecules without the need for a Cu(I) catalyst to result in a stable triazole linkage. The PEG lipophilic spacer helps to reduce aggregation and precipitation problems when labeling biomolecules. Applications Include: Protein-peptide conjugatesAntibody-enzyme or antibody-drug conjugatesProtein or peptide-oligonucleotide conjugatesSurface modification
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
form: solid
reaction suitability: reaction type: click chemistryreagent type: cross-linking reagent
functional group: maleimide
storage temp.: −20°C
SMILES string: O=C(CCNC(CCOCCOCCOCCOCCNC(CCN1C(C=CC1=O)=O)=O)=O)N2CC3=C(C=CC=C3)C#CC4=C2C=CC=C4
InChI: 1S/C36H42N4O9/c41-32(14-18-39-34(43)11-12-35(39)44)38-17-20-47-22-24-49-26-25-48-23-21-46-19-15-33(42)37-16-13-36(45)40-27-30-7-2-1-5-28(30)9-10-29-6-3-4-8-31(29)40/h1-8,11-12H,13-27H2,(H,37,42)(H,38,41)
InChI key: VVFZXPZWVJMYPX-UHFFFAOYSA-N
form:
solid
reaction suitability:
reaction type: click chemistryreagent type: cross-linking reagent
functional group:
maleimide
storage temp.:
−20°C
SMILES string:
O=C(CCNC(CCOCCOCCOCCOCCNC(CCN1C(C=CC1=O)=O)=O)=O)N2CC3=C(C=CC=C3)C#CC4=C2C=CC=C4
InChI:
1S/C36H42N4O9/c41-32(14-18-39-34(43)11-12-35(39)44)38-17-20-47-22-24-49-26-25-48-23-21-46-19-15-33(42)37-16-13-36(45)40-27-30-7-2-1-5-28(30)9-10-29-6-3-4-8-31(29)40/h1-8,11-12H,13-27H2,(H,37,42)(H,38,41)
InChI key:
VVFZXPZWVJMYPX-UHFFFAOYSA-N
form: solid
reaction suitability: reaction type: click chemistryreagent type: cross-linking reagent
functional group: __
storage temp.: −20°C
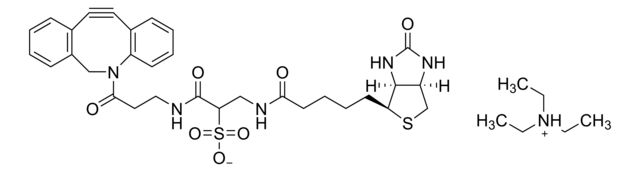
SMILES string: CC[NH+](CC)CC.[O-]S(=O)(=O)C(CNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)C(=O)NCCC(=O)N3Cc4ccccc4C#Cc5ccccc35
InChI: 1S/C31H35N5O7S2.C6H15N/c37-27(12-6-5-11-25-29-23(19-44-25)34-31(40)35-29)33-17-26(45(41,42)43)30(39)32-16-15-28(38)36-18-22-9-2-1-7-20(22)13-14-21-8-3-4-10-24(21)36;1-4-7(5-2)6-3/h1-4,7-10,23,25-26,29H,5-6,11-12,15-19H2,(H,32,39)(H,33,37)(H2,34,35,40)(H,41,42,43);4-6H2,1-3H3/t23-,25-,26?,29-;/m0./s1
InChI key: RACLEVCKGUBCTH-GESOYTMLSA-N
form:
solid
reaction suitability:
reaction type: click chemistryreagent type: cross-linking reagent
functional group:
__
storage temp.:
−20°C
SMILES string:
CC[NH+](CC)CC.[O-]S(=O)(=O)C(CNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)C(=O)NCCC(=O)N3Cc4ccccc4C#Cc5ccccc35
InChI:
1S/C31H35N5O7S2.C6H15N/c37-27(12-6-5-11-25-29-23(19-44-25)34-31(40)35-29)33-17-26(45(41,42)43)30(39)32-16-15-28(38)36-18-22-9-2-1-7-20(22)13-14-21-8-3-4-10-24(21)36;1-4-7(5-2)6-3/h1-4,7-10,23,25-26,29H,5-6,11-12,15-19H2,(H,32,39)(H,33,37)(H2,34,35,40)(H,41,42,43);4-6H2,1-3H3/t23-,25-,26?,29-;/m0./s1
InChI key:
RACLEVCKGUBCTH-GESOYTMLSA-N