324647
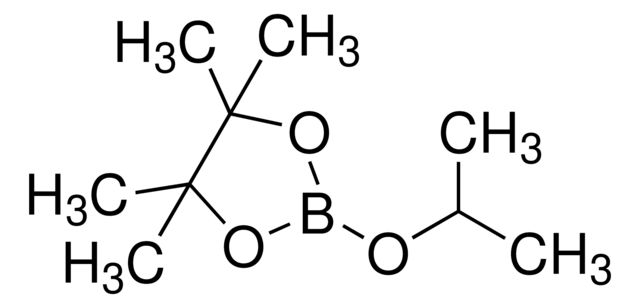
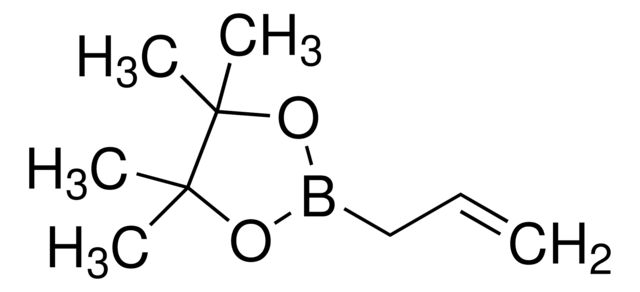
Allylboronic acid pinacol ester
97%
Manufacturer: Sigma Aldrich
CAS Number: 72824-04-5
Synonym(S): 2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4,4,5,5-tetramethyl-2-(2-propen-1-yl)-1,3,2-dioxaborolane, 4,4,5,5-tetramethyl-2-(2-propenyl)-1,3,2-dioxaborolane, Allyl pinacol boronate, Pinacol allylboronate, Pinacolyl 2-propenylboronate
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 324647-1-G | In Stock | ₹ 4,394.95 |
| 10 G | 324647-10-G | In Stock | ₹ 18,121.05 |
324647 - 1 G
In Stock
Quantity
1
Base Price: ₹ 4,394.95
GST (18%): ₹ 791.091
Total Price: ₹ 5,186.041
Quality Level
100
Assay
97%
refractive index
n20/D 1.4268 (lit.)
bp
50-53 °C/5 mmHg (lit.)
density
0.896 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC1(C)OB(CC=C)OC1(C)C
InChI
1S/C9H17BO2/c1-6-7-10-11-8(2,3)9(4,5)12-10/h6H,1,7H2,2-5H3
InChI key
YMHIEPNFCBNQQU-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Aobchem Allylboronic acid pinacol ester, AOBCHEM USA 10686-25G. 72824-04-5. MFCD00013347 | Aobchem | ₹ 9,506.09 | |
 | eMolecules Ambeed / 2-Allyl-4455-tetramethyl-132-dioxaborolane / 1g / 552538916 / A115496 / / 72824-04-5 / MFCD00013347 / 168.040 / C9H17BO2 | eMolecules | ₹ 2,435.04 | |
 | Sigma Aldrich Fine Chemicals Biosciences Allylboronic acid pinacol ester 97% | 72824-04-5 | MFCD00013347 | 10G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 34,561.37 | |
 | 2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane | Aaron Chemicals LLC | ₹ 356.00 - ₹ 36,401.00 | |
 | Allylboronic acid pinacol ester,98%(stabilized with Phenothiazine) | ChemScene | ₹ 2,047.00 - ₹ 9,968.00 |
Related Products
Description
- General description: Allylboronic acid pinacol ester is used as a nucleophile in the catalytic allylation of simple ketoimines. [1]
- Application: Reagent used forPalladium-catalyzed Suzuki-Miyaura cross-coupling reactions and olefin metathesis[2] Intermolecular radical additions[3] Allylboration of aldehydes catalyzed by chiral spirobiindane diol (SPINOL) based phosphoric acids[4] Cobalt-catalyzed regioselective hydrovinylation of dienes with alkenes[5] Nucleic acid-templated energy transfer leading to a photorelease reaction[6] Stereoselective indium-catalyzed Hosomi-Sakurai reactions[7] Reagent used in Preparation ofCyclic sulfone hydroxyethylamines as BACE1 inhibitors for reduction of Amyloid β-Peptides[8] Transmetalation of carbene Ru iodide with Ag carboxylates to give C-H-activated Ru carbene complexes as catalysts for Z-selective olefin metathesis[9] Allylboronation reagent for the preparation of allylic alcohols[10], and homoallylic amines.[11]
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
WGK
WGK 3
Flash Point(F)
closed cup
Flash Point(C)
closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves