7083035
MP Biomedicals™ β-Nicotinamide Adenine Dinucleotide, Oxidized Form, MP Biomedicals™
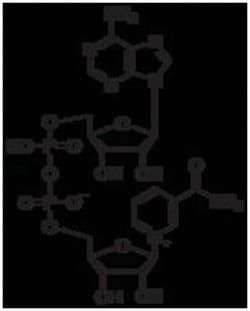
This is an ultrapure NAD, chromatographically purified to remove trace inhibitors.
Manufacturer: Fischer Scientific
The price for this product is unavailable. Please request a quote
Grade
Cell Culture
pH
2 to 4 (1% aq soln)
Molecular Weight (g/mol)
663.4 (anhyd.)
Purity
≥98%
Content And Storage
0°C, Desiccate
Description
- Description β-NAD, a pyridine nucleotide and biologically active form of nicotinic acid, is a coenzyme necessary for the catalytic reaction of certain enzymes
- It occurs in living cells primarily in the oxidized state
- It serves as a coenzyme of the dehydrogenases, especially in the dehydrogenation of primary and secondary alcohols
- NAD usually acts as a hydrogen acceptor, forming NADH which then serves as a hydrogen donor in the respiratory chain
- Electron acceptor
- β-NAD is a carrier for the hydride ion, forming b-NADH
- Hydride ion is enzymatically removed from a substrate molecule by the action of dehydrogenases such as malic dehydrogenase and lactic dehydrogenase
- Such enzymes catalyze the reversible transfer of a hydride ion from malate or lactate to b-NAD to form the reduced product, b-NADH
- Unlike b-NAD which has no absorbance at 340nm, b-NADH absorbs at 340nm (EmM = 6.22)
- The increase in absorbance at 340nm with the formation of b-NADH is the basis for measurement of activity of many enzymes.