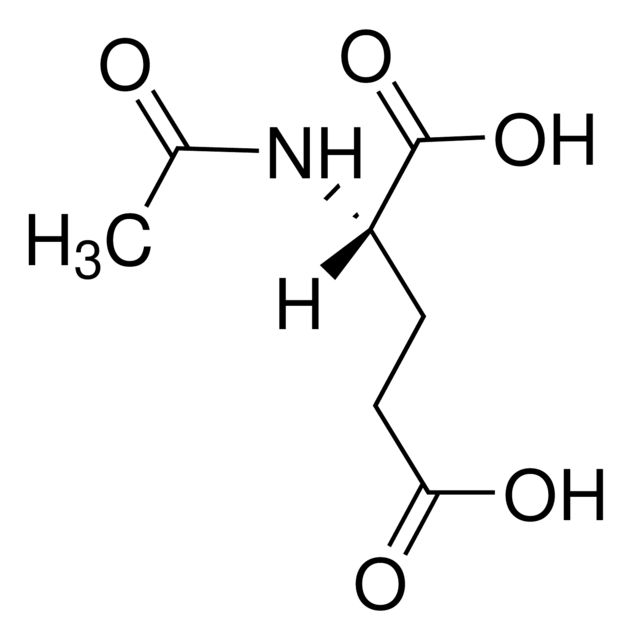
857459
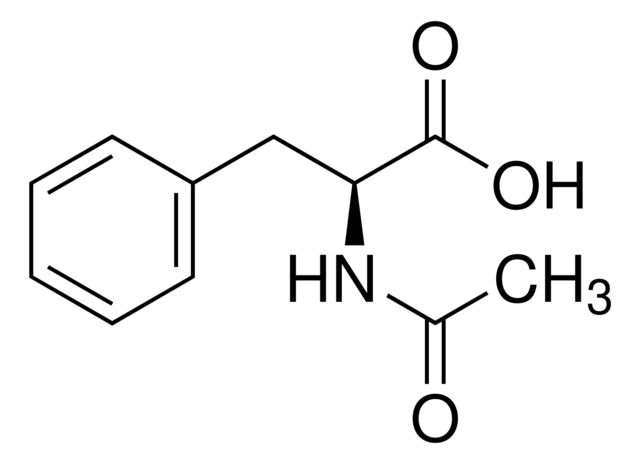
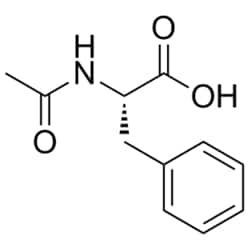
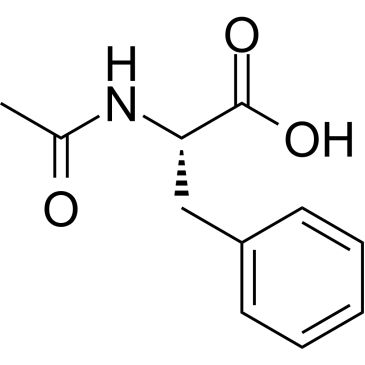
N-Acetyl-L-phenylalanine
ReagentPlus®, 99%
Manufacturer: Sigma Aldrich
CAS Number: 2018-61-3
Synonym(S): (+)-N-Acetylphenylalanine, (S)-2-Acetamido-3-phenylpropanoic acid
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 857459-1-G | In Stock | ₹ 1,991.80 |
| 5 G | 857459-5-G | In Stock | ₹ 4,687.23 |
857459 - 1 G
In Stock
Quantity
1
Base Price: ₹ 1,991.80
GST (18%): ₹ 358.524
Total Price: ₹ 2,350.324
Quality Level
100
product line
ReagentPlus®
Assay
99%
form
powder
optical activity
[α]22/D +40.0°, c = 1 in methanol
reaction suitability
reaction type: C-H Activationreaction type: solution phase peptide synthesisreagent type: ligandreaction type: Peptide Synthesis
mp
171-173 °C (lit.)
application(s)
peptide synthesis
functional group
aminecarboxylic acid
SMILES string
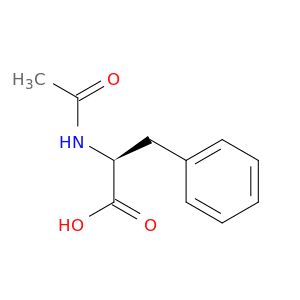
CC(=O)N[C@@H](Cc1ccccc1)C(O)=O
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Medchemexpress LLC HY-Y0068 100mg Medchemexpress, NSC 45699 CAS:2018-61-3 Purity:>98% | Medchemexpress LLC | ₹ 4,672.50 | |
 | N-Acetyl-L-phenylalanine | ChemScene | ₹ 534.00 - ₹ 11,125.00 | |
 | 2018-61-3 | N-Acetyl-L-phenylalanine | A2B Chem | ₹ 445.00 - ₹ 7,298.00 |
Description
- General description: N-Acetyl-L-phenylalanine is an acetyl analog of L-phenylalanine. It is widely used as a reactant to synthesize methyl or ethyl esters of N-acetyl-L-phenylalanine, which are employed as versatile building blocks in peptide synthesis.[1][2]
- Application: N-Acetyl-L-phenylalanine can be used as a reactant to synthesize:N-acetyl phenylalanine methyl ester by esterification reaction with methanol using Mukaiyama′s reagent.[2]Acetylaminocyclohexane propanoic acid by rhodium-catalyzed hydrogenation reaction.[3]
- Legal Information: ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
Quality Level: 100
product line: ReagentPlus®
Assay: 99%
form: powder
optical activity: [α]22/D +40.0°, c = 1 in methanol
reaction suitability: reaction type: C-H Activationreaction type: solution phase peptide synthesisreagent type: ligandreaction type: Peptide Synthesis
mp: 171-173 °C (lit.)
application(s): peptide synthesis
functional group: aminecarboxylic acid
SMILES string: CC(=O)N[C@@H](Cc1ccccc1)C(O)=O
Quality Level:
100
product line:
ReagentPlus®
Assay:
99%
form:
powder
optical activity:
[α]22/D +40.0°, c = 1 in methanol
reaction suitability:
reaction type: C-H Activationreaction type: solution phase peptide synthesisreagent type: ligandreaction type: Peptide Synthesis
mp:
171-173 °C (lit.)
application(s):
peptide synthesis
functional group:
aminecarboxylic acid
SMILES string:
CC(=O)N[C@@H](Cc1ccccc1)C(O)=O
Quality Level: __
product line: __
Assay: >99% (TLC)
form: powder
optical activity: __
reaction suitability: __
mp: __
application(s): __
functional group: __
SMILES string: [H][C@@](COCCC([N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])C([O-])=O)(OC(CCCCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCCCC)=O
Quality Level:
__
product line:
__
Assay:
>99% (TLC)
form:
powder
optical activity:
__
reaction suitability:
__
mp:
__
application(s):
__
functional group:
__
SMILES string:
[H][C@@](COCCC([N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])C([O-])=O)(OC(CCCCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCCCC)=O