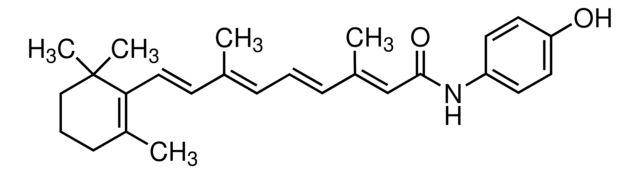
Retinoic acid p-hydroxyanilide
≥95%
Manufacturer: Sigma Aldrich
CAS Number: 65646-68-6
Synonym(S): 4-HPR, Fenretinide, N-(4-Hydroxyphenyl)retinamide
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 5 MG | H7779-5-MG | In Stock | ₹ 24,745.95 |
H7779 - 5 MG
In Stock
Quantity
1
Base Price: ₹ 24,745.95
GST (18%): ₹ 4,454.271
Total Price: ₹ 29,200.221
biological source
synthetic (organic)
Quality Level
200
Assay
≥95%
form
powder
color
yellow to yellow-orange
storage temp.
−20°C
SMILES string
CC1=C(\C=C\C(C)=C\C=C\C(C)=C\C(=O)Nc2ccc(O)cc2)C(C)(C)CCC1
InChI
1S/C26H33NO2/c1-19(11-16-24-21(3)10-7-17-26(24,4)5)8-6-9-20(2)18-25(29)27-22-12-14-23(28)15-13-22/h6,8-9,11-16,18,28H,7,10,17H2,1-5H3,(H,27,29)/b9-6+,16-11+,19-8+,20-18+
InChI key
AKJHMTWEGVYYSE-FXILSDISSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Selleck Chemical LLC Fenretinide 25mg 65646-68-6 4-hydroxy(phenyl)retinamide, 4-HPR, MK-4016 | Selleck Chemical LLC | ₹ 16,767.60 | |
 | Apexbio Technology LLC Fenretinide, 10mg. Cas: 65646-68-6 MFCD: MFCD00792674. Synthetic retinoid agonist | Apexbio Technology LLC | ₹ 4,717.00 | |
 | MilliporeSigma™ Calbiochem™ 4-Hydroxyphenylretinamide | MilliporeSigma™ | ₹ 20,855.37 | |
 | Enzo Life Sciences 4-Hydroxyphenylretinamide (5mg). CAS: 65646-68-6 | Enzo Life Sciences | ₹ 14,840.75 | |
 | eMolecules Fenretinide | 65646-68-6 | MFCD00792674 | 100mg | eMolecules | ₹ 27,225.99 | |
 | Sigma Aldrich Fine Chemicals Biosciences Retinoic acid p-hydroxyanilide >=95% | 65646-68-6 | MFCD00792674 | 5MG | Sigma Aldrich Fine Chemicals Biosciences | ₹ 23,767.45 | |
 | Biotang Inc Fenretinide, > 99%, 50MG, 4-HPR;MK-4016;McN-R-1967;RII retinamide;Ro 22-4667. M.W. 391.55, C26H33NO2, 65646-68-6. | Biotang Inc | ₹ 25,979.10 |
Description
- General description: Retinoic acid p-hydroxyanilide is a synthetic analog of retinoid.[1]
- Application: Retinoic acid p-hydroxyanilide has been used: as a synthetic retinoid to induce apoptosis in SEB-1 sebocytes[1]as a medium supplement for C2C12 myoblasts to test its effect on ceramide formation[2] to test in cytotoxicity in T-cell acute lymphoblastic leukemia (T-ALL)[3]
- Biochem/physiol Actions: Retinoic acid p-hydroxyanilide, also called fenretinide, increases reactive oxygen species, activates caspases and induces apoptosis.[1] It also inhibits dihydroceramide desaturase, leading to a decrease in ceramide biosynthesis.[2] Fenretinide may elicit anticancer activity in cultured human breast cancer cells.[2] It acts as an insulin antagonist and may be useful in treating insulin resistance.[2] Fenretinide or 4-HPR has chemotherapeutic potential and is cytotoxic to retinoic acid-resistant cancers.[3]
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
P280 - P301 + P312 - P302 + P352 + P312 - P304 + P340 + P312 - P305 + P351 + P338 - P308 + P313
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves