921300
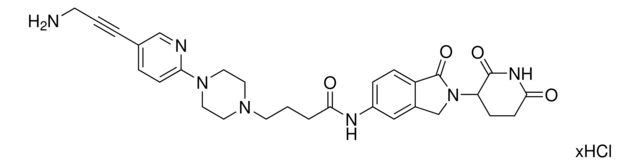
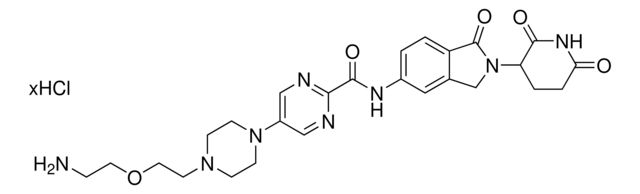
C5 Lenalidomide-pyrimidine-piperazine-PEG1-NH2 hydrochloride
≥95%
Manufacturer: Sigma Aldrich
Synonym(S): 5-(4-(2-(2-Aminoethoxy)ethyl)piperazin-1-yl)-N-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-5-yl)pyrimidine-2-carboxamide hydrochloride, Crosslinker−E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 50 MG | 921300-50-MG | In Stock | ₹ 53,042.50 |
921300 - 50 MG
In Stock
Quantity
1
Base Price: ₹ 53,042.50
GST (18%): ₹ 9,547.65
Total Price: ₹ 62,590.15
ligand
C5 Lenalidomide
Quality Level
100
Assay
≥95%
form
(Powder or crystals or flakes or chunks)
reaction suitability
reactivity: carboxyl reactivereagent type: ligand-linker conjugate
functional group
amine
storage temp.
2-8°C
SMILES string
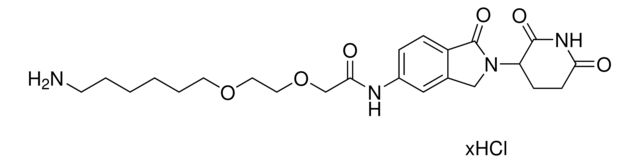
O=C1N(C2CCC(NC2=O)=O)CC3=CC(NC(C4=NC=C(N5CCN(CCOCCN)CC5)C=N4)=O)=CC=C31.Cl
Related Products
Description
- Application: Protein degrader builiding block C5 Lenalidomide-pyrimidine-piperazine-PEG1-NH2 hydrochloride enables the synthesis of molecules for targeted protein degradation and PROTAC (proteolysis-targeting chimeras) technology. This conjugate contains a Cereblon (CRBN)-recruiting ligand with alternative exit vector, a linker with added rigidity, and a pendant amine for reactivity with a carboxylic acid on the target ligand. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and PROTAC, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a terminal amine, parallel synthesis can be used to more quickly generate PROTAC libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
- Other Notes: Technology Spotlight: Degrader Building Blocks for Targeted Protein DegradationPortal: Building PROTAC® Degraders for Targeted Protein Degradation Targeted Protein Degradation by Small MoleculesSmall-Molecule PROTACS: New Approaches to Protein DegradationTargeted Protein Degradation: from Chemical Biology to Drug DiscoveryImpact of linker length on the activity of PROTACs
- Legal Information: PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
ligand: C5 Lenalidomide
Quality Level: 100
Assay: ≥95%
form: (Powder or crystals or flakes or chunks)
reaction suitability: reactivity: carboxyl reactivereagent type: ligand-linker conjugate
functional group: amine
storage temp.: 2-8°C
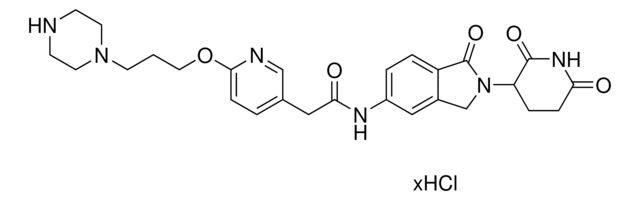
SMILES string: O=C1N(C2CCC(NC2=O)=O)CC3=CC(NC(C4=NC=C(N5CCN(CCOCCN)CC5)C=N4)=O)=CC=C31.Cl
ligand:
C5 Lenalidomide
Quality Level:
100
Assay:
≥95%
form:
(Powder or crystals or flakes or chunks)
reaction suitability:
reactivity: carboxyl reactivereagent type: ligand-linker conjugate
functional group:
amine
storage temp.:
2-8°C
SMILES string:
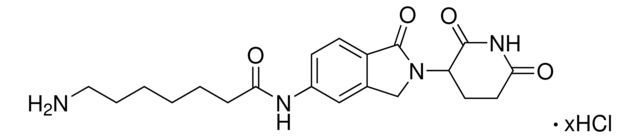
O=C1N(C2CCC(NC2=O)=O)CC3=CC(NC(C4=NC=C(N5CCN(CCOCCN)CC5)C=N4)=O)=CC=C31.Cl
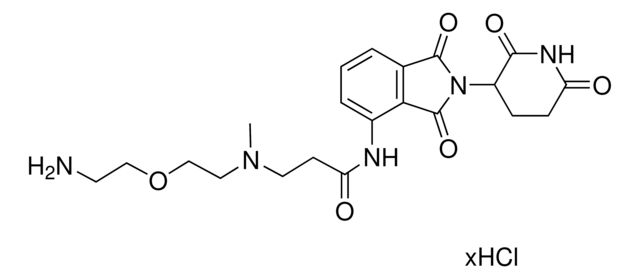
ligand: pomalidomide
Quality Level: 100
Assay: __
form: (Powder or Crystals or Solid or Chunks)
reaction suitability: reactivity: carboxyl reactivereagent type: ligand-linker conjugate
functional group: amine
storage temp.: 2-8°C
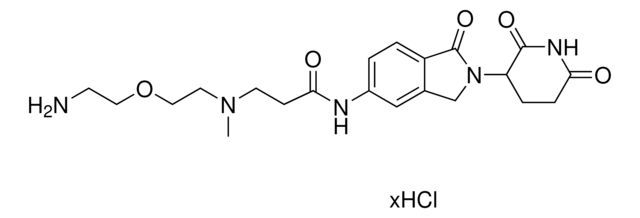
SMILES string: O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(CCN(C)CCOCCN)=O)=O)NC1=O.Cl
ligand:
pomalidomide
Quality Level:
100
Assay:
__
form:
(Powder or Crystals or Solid or Chunks)
reaction suitability:
reactivity: carboxyl reactivereagent type: ligand-linker conjugate
functional group:
amine
storage temp.:
2-8°C
SMILES string:
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(CCN(C)CCOCCN)=O)=O)NC1=O.Cl