BDB556449
BD Ac-DEVD-AMC Caspase-3 Substrate
Manufacturer: BD
The price for this product is unavailable. Please request a quote
Form
Lyophilized
Substrate Type
Caspase Substrate
Molecular Weight (g/mol)
694
Substrate Quantity
1mg
For Use With (Application)
For Caspase-3, fluorescence quantitation, used in protease assays
Includes
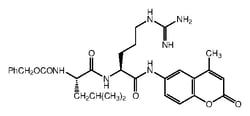
Amino acid sequence of the PARP cleavage site at Asp 216
Storage Requirements
-20°C
Description
- Members of the ICE/CED-3 cysteine protease family have key roles in inflammation and mammalian apoptosis
- The ICE family member Caspase-3 (also known as CPP32, Yama, apopain) is activated early in apoptosis and appears to be involved in the proteolysis of several important molecules, including poly (ADP ribose) polymerase (PARP)
- Activated Caspase-3 cleaves PARP from its 116 kDa to an 85 kDa residual fragment
- The cleavage site in PARP is C-terminal to Asp-216
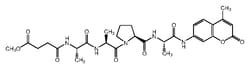
- The upstream sequence of the cleavage site, DEVD (Asp-Glu-Val-Asp), is utilized as a basis for the highly specific Caspase-3 substrate, Ac (N-acetyl)-DEVD-AMC (7-amino-4-methylcoumarin)
- Format: Purified Storage buffer: Lyophilized powder Functional assay
SAFETY INFORMATION
- ShelfLife : 1 to 2 Months