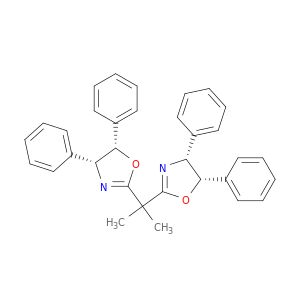
795682
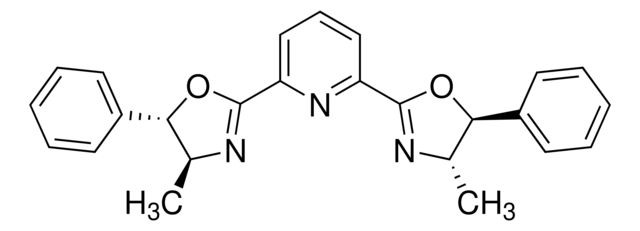
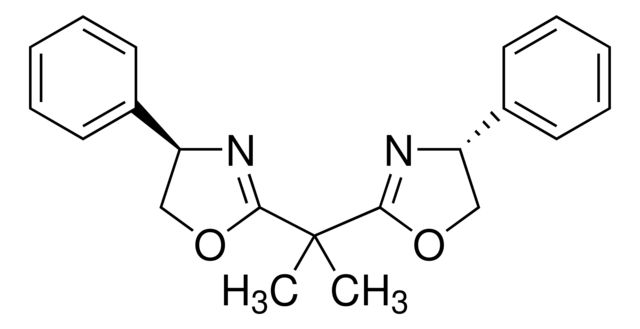
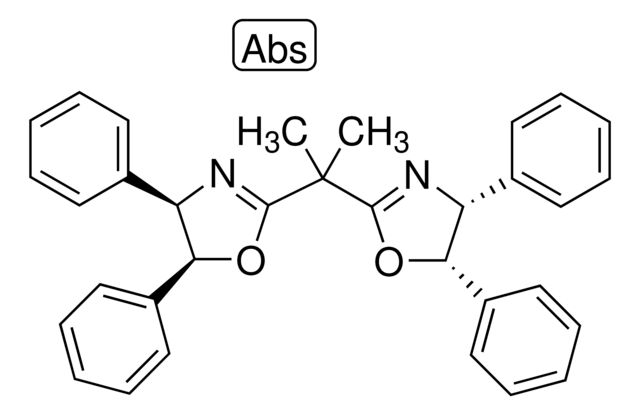
(4R,4′R,5S,5′S)-2,2′-(1-Methylethylidene
95%
Manufacturer: Sigma Aldrich
CAS Number: 157904-67-1
Synonym(S): 2,2-Bis[2-(4R,5S-diphenyl-1,3-oxazolinyl)]propane
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 250 MG | 795682-250-MG | In Stock | ₹ 21,747.43 |
795682 - 250 MG
In Stock
Quantity
1
Base Price: ₹ 21,747.43
GST (18%): ₹ 3,914.537
Total Price: ₹ 25,661.967
Quality Level
100
Assay
95%
form
solid
mp
164-169 °C
SMILES string
CC(C1=N[C@H](C2=CC=CC=C2)[C@H](C3=CC=CC=C3)O1)(C)C4=N[C@H](C5=CC=CC=C5)[C@H](C6=CC=CC=C6)O4
InChI
1S/C33H30N2O2/c1-33(2,31-34-27(23-15-7-3-8-16-23)29(36-31)25-19-11-5-12-20-25)32-35-28(24-17-9-4-10-18-24)30(37-32)26-21-13-6-14-22-26/h3-22,27-30H,1-2H3/t27-,28-,29+,30+/m1/s1
InChI key
ZWWGNCSTEMMQOQ-XAZDILKDSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences (4R,4'R,5S,5'S)-2,2'-(1-Methylethylidene 95% | Purity: 95% | Mol Wt: 486.6 | 157904-67-1 | MFCD24849939 | 250MG | Sigma Aldrich Fine Chemicals Biosciences | ₹ 32,598.92 | |
 | Oxazole, 2,2'-(1-methylethylidene)bis[4,5-dihydro-4,5-diphenyl-, (4R,4'R,5S,5'S)- | Aaron Chemicals LLC | ₹ 1,424.00 - ₹ 1,12,051.00 | |
 | 157904-67-1 | (4R,4'R,5S,5'S)-2,2'-(1-Methylethylidene)bis[4,5-dihydro-4,5-diphenyloxazole | A2B Chem | ₹ 2,225.00 - ₹ 21,449.00 |
Related Products
Description
- Application: (4R,4′R,5S,5′S)-2,2′-(1-Methylethylidene) or (4S,5R)-Bis-Phbox) can be used as a ligand:To prepare selective exo-catalysts for enantioselective 1,3-dipolar cycloaddition reactions.[1] In the asymmetric aminooxygenation of alkenes in the presence of tetramethylaminopyridyl radical (TEMPO) as an oxidant and copper(II) triflate as a catalyst.[2]In asymmetric aminofluorination of olefins using an iron catalyst.[3]This chiral Box ligand was most recently shown to mediate an asymmetric aminofluorination of olefins utlizing Xtalfuor-E (719439) and TREAT-HF (344648). The resulting cyclic carbamates can be readily converted into their concomitant beta-fluoro amino acids.
- Other Notes: Iron(II)-Catalyzed Intramolecular Olefin Aminofluorination
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
Quality Level: 100
Assay: 95%
form: solid
mp: 164-169 °C
SMILES string: CC(C1=N[C@H](C2=CC=CC=C2)[C@H](C3=CC=CC=C3)O1)(C)C4=N[C@H](C5=CC=CC=C5)[C@H](C6=CC=CC=C6)O4
InChI: 1S/C33H30N2O2/c1-33(2,31-34-27(23-15-7-3-8-16-23)29(36-31)25-19-11-5-12-20-25)32-35-28(24-17-9-4-10-18-24)30(37-32)26-21-13-6-14-22-26/h3-22,27-30H,1-2H3/t27-,28-,29+,30+/m1/s1
InChI key: ZWWGNCSTEMMQOQ-XAZDILKDSA-N
Quality Level:
100
Assay:
95%
form:
solid
mp:
164-169 °C
SMILES string:
CC(C1=N[C@H](C2=CC=CC=C2)[C@H](C3=CC=CC=C3)O1)(C)C4=N[C@H](C5=CC=CC=C5)[C@H](C6=CC=CC=C6)O4
InChI:
1S/C33H30N2O2/c1-33(2,31-34-27(23-15-7-3-8-16-23)29(36-31)25-19-11-5-12-20-25)32-35-28(24-17-9-4-10-18-24)30(37-32)26-21-13-6-14-22-26/h3-22,27-30H,1-2H3/t27-,28-,29+,30+/m1/s1
InChI key:
ZWWGNCSTEMMQOQ-XAZDILKDSA-N