900602
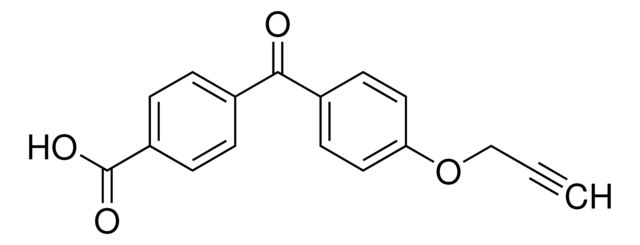
4-(4-(Prop-2-yn-1-yloxy)benzoyl)benzoic acid
≥95%
Manufacturer: Sigma Aldrich
CAS Number: 1236196-77-2
Synonym(S): Carboxyl benzophenone alkyne, Probe building block
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 100 MG | 900602-100-MG | In Stock | ₹ 23,089.73 |
900602 - 100 MG
In Stock
Quantity
1
Base Price: ₹ 23,089.73
GST (18%): ₹ 4,156.151
Total Price: ₹ 27,245.881
Quality Level
100
Assay
≥95%
form
solid
reaction suitability
reaction type: click chemistryreagent type: cross-linking reagent
functional group
alkynecarboxylic acid
storage temp.
−20°C
SMILES string
O=C(C1=CC=C(OCC#C)C=C1)C2=CC=C(C(O)=O)C=C2
InChI
1S/C17H12O4/c1-2-11-21-15-9-7-13(8-10-15)16(18)12-3-5-14(6-4-12)17(19)20/h1,3-10H,11H2,(H,19,20)
InChI key
URDMKFAPEKSQDV-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
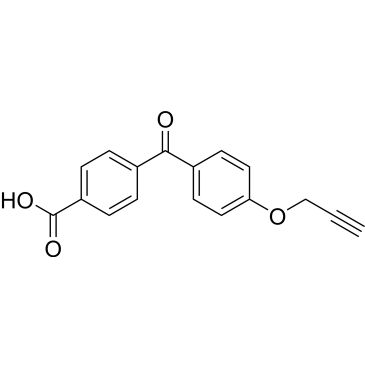 | 4-(4-(Prop-2-yn-1-yloxy)benzoyl)benzoic acid | ChemScene | ₹ 12,104.00 - ₹ 53,845.00 | |
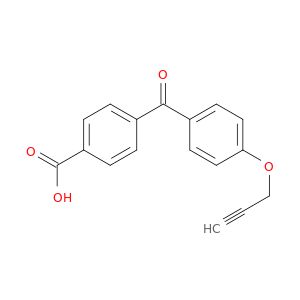 | 1236196-77-2 | 4-(4-(prop-2-ynyloxy)benzoyl)benzoic acid | A2B Chem | ₹ 9,701.00 - ₹ 19,847.00 |
Related Products
Description
- Application: 4-(4-(Prop-2-yn-1-yloxy)benzoyl)benzoic acid is used for chemical probe synthesis, this trifunctional building block contains a light-activated benzophenone, alkyne tag, and carboxylic acid synthetic handle. When appended to a ligand or pharmacophore through its acid linker, this building block allows for UV light-induced covalent modification of a biological target with potential for downstream applications via the alkyne tag. Use alone or in parallel with other multi-functional building blocks to discover the optimal probe for your chemical biology experiments.
- Other Notes: Technology Spotlight: Trifuctional Probe Building BlocksA library approach to rapidly discover photoaffinity probes of the mRNA decapping scavenger enzyme DcpSSynthesis and Evaluation of New Thiodigalactoside-Based Chemical Probes to Label Galectin-3Discovery of tumor-specific irreversible inhibitors of stearoyl CoA desaturase
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
Quality Level: 100
Assay: ≥95%
form: solid
reaction suitability: reaction type: click chemistryreagent type: cross-linking reagent
functional group: alkynecarboxylic acid
storage temp.: −20°C
SMILES string: O=C(C1=CC=C(OCC#C)C=C1)C2=CC=C(C(O)=O)C=C2
InChI: 1S/C17H12O4/c1-2-11-21-15-9-7-13(8-10-15)16(18)12-3-5-14(6-4-12)17(19)20/h1,3-10H,11H2,(H,19,20)
InChI key: URDMKFAPEKSQDV-UHFFFAOYSA-N
Quality Level:
100
Assay:
≥95%
form:
solid
reaction suitability:
reaction type: click chemistryreagent type: cross-linking reagent
functional group:
alkynecarboxylic acid
storage temp.:
−20°C
SMILES string:
O=C(C1=CC=C(OCC#C)C=C1)C2=CC=C(C(O)=O)C=C2
InChI:
1S/C17H12O4/c1-2-11-21-15-9-7-13(8-10-15)16(18)12-3-5-14(6-4-12)17(19)20/h1,3-10H,11H2,(H,19,20)
InChI key:
URDMKFAPEKSQDV-UHFFFAOYSA-N
Quality Level: 100
Assay: 95%
form: powder or crystals
reaction suitability: core: palladiumreagent type: catalystreaction type: Cross Couplings
functional group: __
storage temp.: __
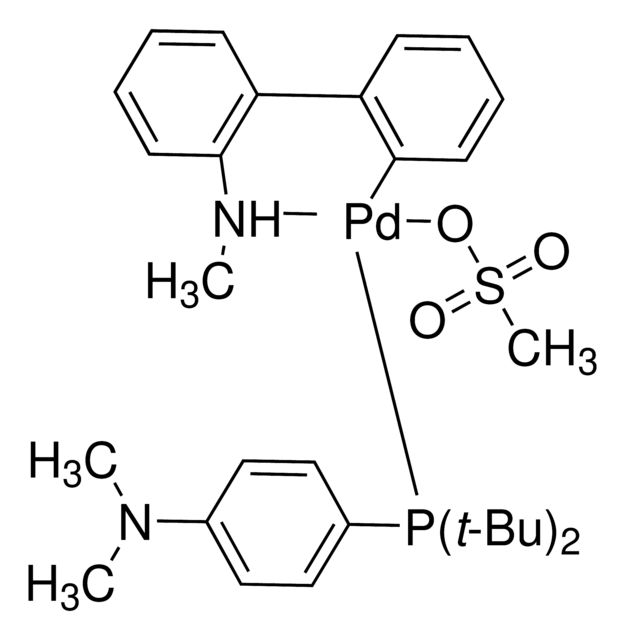
SMILES string: O=S(=O)([O-][Pd+2]1([C-]=2C=CC=CC2C=3C=CC=CC3[NH]1C)[P](C4=CC=C(C=C4)N(C)C)(C(C)(C)C)C(C)(C)C)C
InChI: 1S/C16H28NP.C13H12N.CH4O3S.Pd/c1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;1-14-13-10-6-5-9-12(13)11-7-3-2-4-8-11;1-5(2,3)4;/h9-12H,1-8H3;2-7,9-10,14H,1H3;1H3,(H,2,3,4);/q;-1;;+2/p-1
InChI key: MLEINNWTBGCQPS-UHFFFAOYSA-M
Quality Level:
100
Assay:
95%
form:
powder or crystals
reaction suitability:
core: palladiumreagent type: catalystreaction type: Cross Couplings
functional group:
__
storage temp.:
__
SMILES string:
O=S(=O)([O-][Pd+2]1([C-]=2C=CC=CC2C=3C=CC=CC3[NH]1C)[P](C4=CC=C(C=C4)N(C)C)(C(C)(C)C)C(C)(C)C)C
InChI:
1S/C16H28NP.C13H12N.CH4O3S.Pd/c1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;1-14-13-10-6-5-9-12(13)11-7-3-2-4-8-11;1-5(2,3)4;/h9-12H,1-8H3;2-7,9-10,14H,1H3;1H3,(H,2,3,4);/q;-1;;+2/p-1
InChI key:
MLEINNWTBGCQPS-UHFFFAOYSA-M