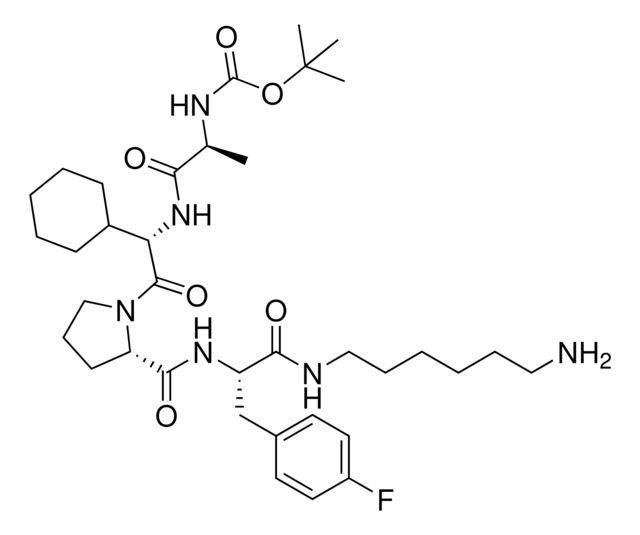
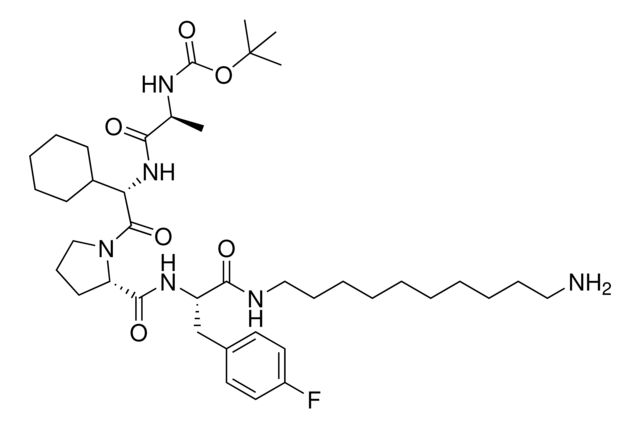
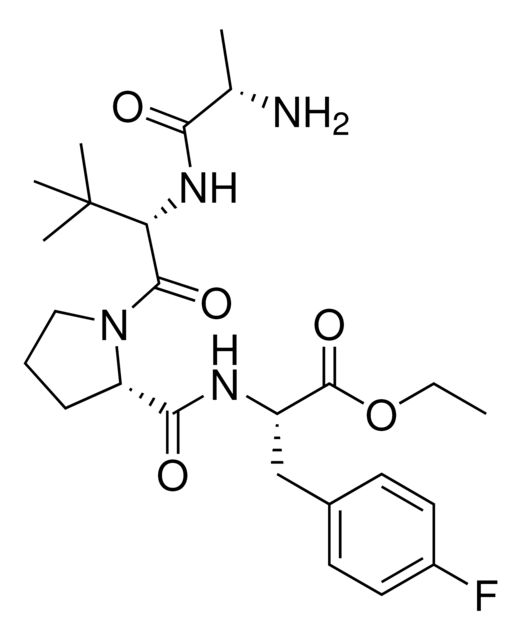
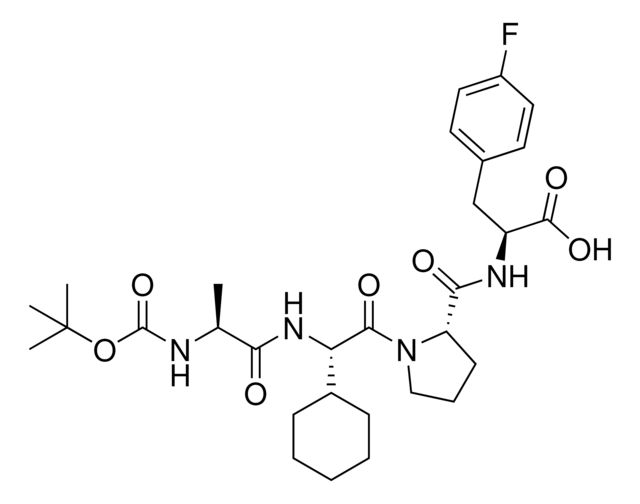
917974
BocA1V2PF2
Manufacturer: Sigma Aldrich
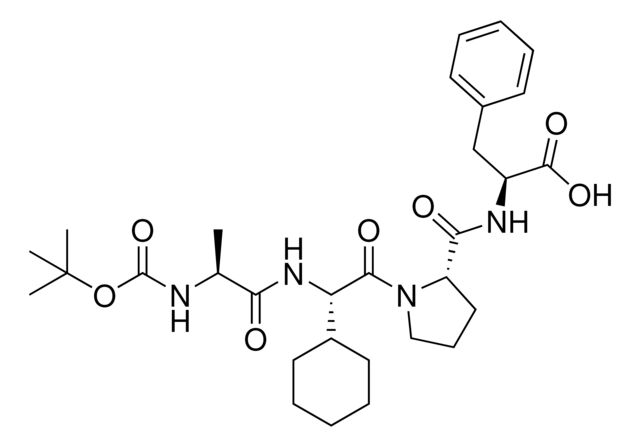
Synonym(S): (S)-2-((S)-1-((S)-2-((S)-2-((tert-Butoxycarbonyl)amino)propanamido)-2-cyclohexylacetyl)pyrrolidine-2-carboxamido)-3-(4-fluorophenyl)propanoic acid, IAP E3 ligase lead for protein degrader research, SNIPER building block
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 100 MG | 917974-100-MG | In Stock | ₹ 46,839.78 |
917974 - 100 MG
In Stock
Quantity
1
Base Price: ₹ 46,839.78
GST (18%): ₹ 8,431.16
Total Price: ₹ 55,270.94
ligand
BocA1V2PF2
Quality Level
100
form
powder
reaction suitability
reagent type: ligand
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
C[C@H](NC(OC(C)(C)C)=O)C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(O)=O)CC2=CC=C(C=C2)F)=O)=O)C3CCCCC3)=O
Related Products
Description
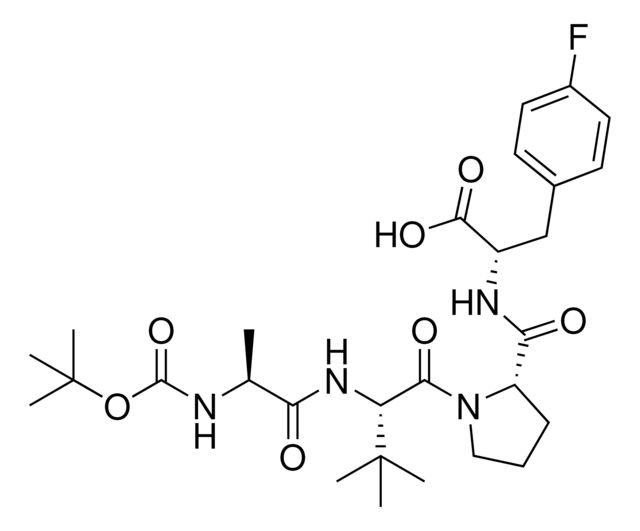
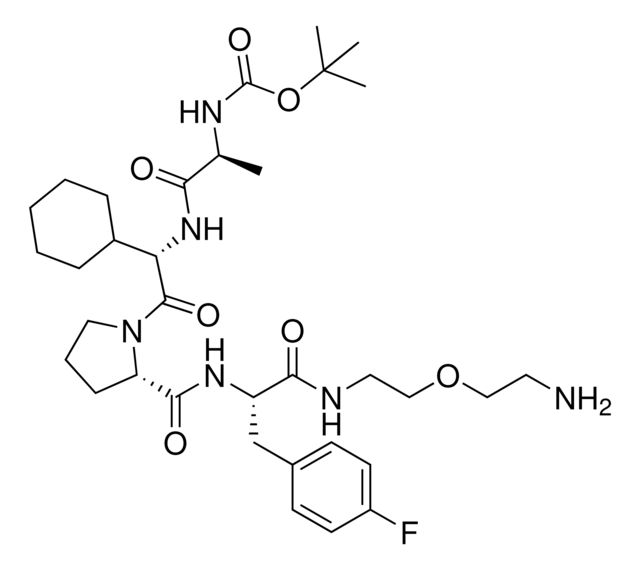
- Application: BocA1V2PF2 is an in silico-derived inhibitor of apoptosis protein (IAP)-recruiting ligand for targeted protein degradation and SNIPER (specific and non-genetic IAP-dependent protein erasers) development, launched in partnership with ComInnex. Learn more about the novel IAP ligands generated through virtual screening of AVP mimetics in our Technology Spotlights. A C-terminal variant of BocA1V2PF2 is also available as A1V2PF2-NHEt (916714).BocA1V2PF2 conjugates are also available for degrader synthesis. Browse our full synthesis offering here for streamlining SNIPER and PROTAC® degrader libraries: Degrader Building Blocks917443BocA1V2PF2-NHC6-NH2917699 BocA1V2PF2-NHC10-NH2917958 BocA1V2PF2-NHPEG1-NH2916692 BocA1V2PF2-NHPEG3-NH2
- Other Notes: In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs) SNIPERs—Hijacking IAP activity to induce protein degradation E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones
- Legal Information: PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable