L2906
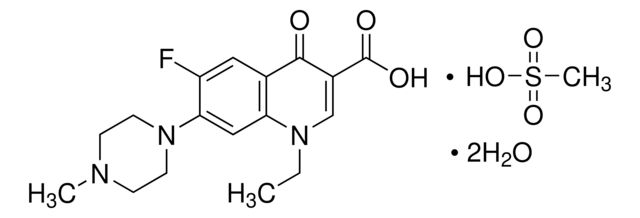
Lomefloxacin hydrochloride
Manufacturer: Sigma Aldrich
CAS Number: 98079-52-8
Synonym(S): 1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | L2906-1-G | In Stock | ₹ 7,015.20 |
| 10 G | L2906-10-G | In Stock | ₹ 39,782.40 |
L2906 - 1 G
In Stock
Quantity
1
Base Price: ₹ 7,015.20
GST (18%): ₹ 1,262.736
Total Price: ₹ 8,277.936
biological source
synthetic
Quality Level
200
color
white to off-white
antibiotic activity spectrum
Gram-negative bacteriaGram-positive bacteria
Mode of action
DNA synthesis | interferesenzyme | inhibits
storage temp.
−20°C
SMILES string
Cl.CCN1C=C(C(O)=O)C(=O)c2cc(F)c(N3CCNC(C)C3)c(F)c12
InChI
1S/C17H19F2N3O3.ClH/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22;/h6,8-9,20H,3-5,7H2,1-2H3,(H,24,25);1H
InChI key
KXEBLAPZMOQCKO-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences Lomefloxacin hydrochloride | 98079-52-8 | MFCD00214312 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 8,641.90 | |
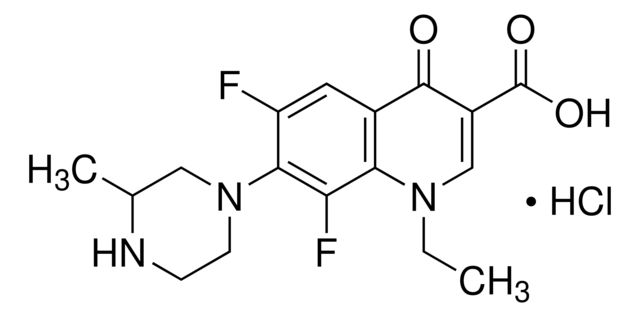 | Lomefloxacin hydrochloride | Sigma Aldrich | ₹ 7,015.20 - ₹ 39,782.40 | |
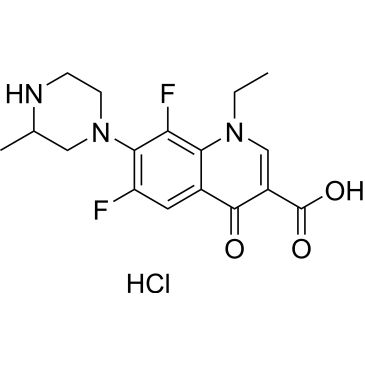 | Lomefloxacin hydrochloride | ChemScene | ₹ 5,073.00 |
Related Products
Description
- General description: Chemical structure: fluoroquinolone
- Application: Lomefloxacin is a fluoroquinolone antibiotic that is commonly used to treat bacterial infections, including bronchitis and urinary tract infections. It is used as a pre-operative prophylactic to prevent urinary tract infection caused by S. pneumoniae, H. influenzae, S. aureus, P. aeruginosa, E. cloacae, P. mirabilis, C. civersus, S. asprphyticus, E. coli, and K. pneumoniae. It is used to induce genomic instability in mice[1] and modification of the kinetics of growth of Gram-negative bacteria[2].
- Biochem/physiol Actions: Lomefloxacin is a bactericidal fluoroquinolone agent that is active against gram-negative and gram-positive organisms. Lomefloxacin inhibits bacterial DNA gyrase (topoisomerase II) and topoisomerase IV, which are needed for the transcription and replication of bacterial DNA. DNA gyrase is thought to be the primary quinolone target for gram-negative bacteria. Topoisomerase IV is thought to be the primary target in gram-positive organisms. The inhibition of the topoisomerases results in strand breakage of the bacterial chromosome, supercoiling, and resealing. Therefore, DNA replication and transcription is inhibited.
SAFETY INFORMATION
Compare Similar Items
Show Difference
biological source: synthetic
Quality Level: 200
color: white to off-white
antibiotic activity spectrum: Gram-negative bacteriaGram-positive bacteria
Mode of action: DNA synthesis | interferesenzyme | inhibits
storage temp.: −20°C
SMILES string: Cl.CCN1C=C(C(O)=O)C(=O)c2cc(F)c(N3CCNC(C)C3)c(F)c12
InChI: 1S/C17H19F2N3O3.ClH/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22;/h6,8-9,20H,3-5,7H2,1-2H3,(H,24,25);1H
InChI key: KXEBLAPZMOQCKO-UHFFFAOYSA-N
biological source:
synthetic
Quality Level:
200
color:
white to off-white
antibiotic activity spectrum:
Gram-negative bacteriaGram-positive bacteria
Mode of action:
DNA synthesis | interferesenzyme | inhibits
storage temp.:
−20°C
SMILES string:
Cl.CCN1C=C(C(O)=O)C(=O)c2cc(F)c(N3CCNC(C)C3)c(F)c12
InChI:
1S/C17H19F2N3O3.ClH/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22;/h6,8-9,20H,3-5,7H2,1-2H3,(H,24,25);1H
InChI key:
KXEBLAPZMOQCKO-UHFFFAOYSA-N
biological source: __
Quality Level: 300
color: __
antibiotic activity spectrum: fungi
Mode of action: protein synthesis | interferes
storage temp.: −20°C
SMILES string: CCC(\C=C\[C@@H]1OC(=O)C=C[C@@H]1C)=C\[C@H](C)C\C=C\C(C)=C\[C@@H](C)C(=O)[C@@H](C)[C@H](O)[C@@H](C)C\C(C)=C\C(O)=O
InChI: 1S/C33H48O6/c1-9-28(14-15-29-24(5)13-16-31(36)39-29)19-22(3)12-10-11-21(2)17-25(6)32(37)27(8)33(38)26(7)18-23(4)20-30(34)35/h10-11,13-17,19-20,22,24-27,29,33,38H,9,12,18H2,1-8H3,(H,34,35)/b11-10+,15-14+,21-17+,23-20+,28-19-/t22-,24+,25-,26+,27-,29+,33-/m1/s1
InChI key: __
biological source:
__
Quality Level:
300
color:
__
antibiotic activity spectrum:
fungi
Mode of action:
protein synthesis | interferes
storage temp.:
−20°C
SMILES string:
CCC(\C=C\[C@@H]1OC(=O)C=C[C@@H]1C)=C\[C@H](C)C\C=C\C(C)=C\[C@@H](C)C(=O)[C@@H](C)[C@H](O)[C@@H](C)C\C(C)=C\C(O)=O
InChI:
1S/C33H48O6/c1-9-28(14-15-29-24(5)13-16-31(36)39-29)19-22(3)12-10-11-21(2)17-25(6)32(37)27(8)33(38)26(7)18-23(4)20-30(34)35/h10-11,13-17,19-20,22,24-27,29,33,38H,9,12,18H2,1-8H3,(H,34,35)/b11-10+,15-14+,21-17+,23-20+,28-19-/t22-,24+,25-,26+,27-,29+,33-/m1/s1
InChI key:
__