PHR1497
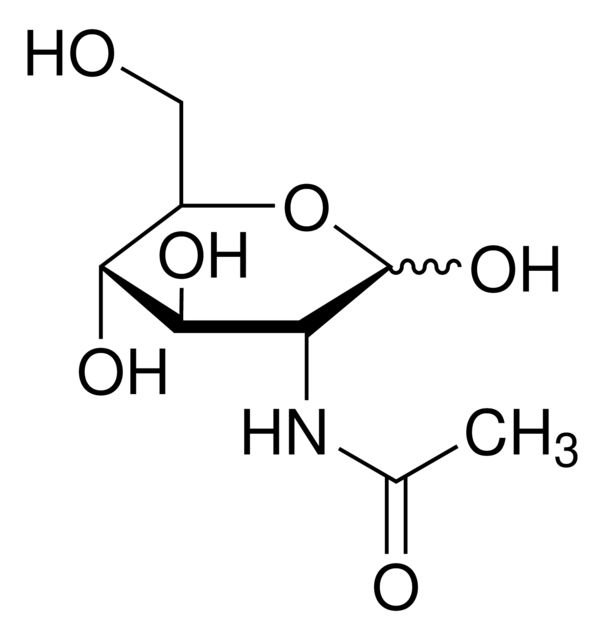
Maltose Monohydrate
Pharmaceutical Secondary Standard; Certified Reference Material
Manufacturer: Supelco
CAS Number: 6363-53-7
Synonym(S): D-Maltose monohydrate, 4-O-α-D-Glucopyranosyl-D-glucose, D-(+)-Maltose monohydrate, Maltobiose
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | PHR1497-1-G | In Stock | ₹ 13,286.70 |
PHR1497 - 1 G
In Stock
Quantity
1
Base Price: ₹ 13,286.70
GST (18%): ₹ 2,391.606
Total Price: ₹ 15,678.306
grade
certified reference materialpharmaceutical secondary standard
Quality Level
300
Agency
traceable to USP 1375025
API family
maltose
CofA
current certificate can be downloaded
packaging
pkg of 1 g
technique(s)
HPLC: suitablegas chromatography (GC): suitable
mp
119-121 °C (dec.) (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
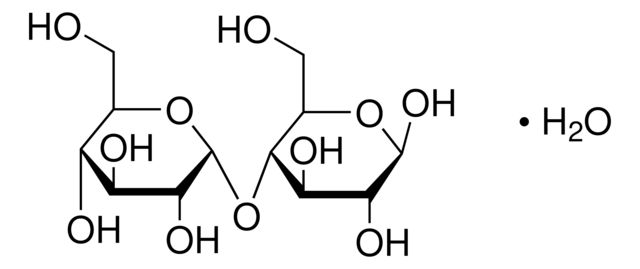 | D-(+)-Maltose monohydrate | Supelco | ₹ 4,415.60 | |
 | Maltose monohydrate | Sigma Aldrich | ₹ 67,377.80 | |
 | D-(+)-Maltose monohydrate | Sigma Aldrich | ₹ 7,128.80 - ₹ 68,707.80 | |
 | D-(+)-Maltose monohydrate | Sigma Aldrich | ₹ 3,245.20 - ₹ 18,806.20 | |
 | D-(+)-Maltose monohydrate | Sigma Aldrich | ₹ 22,210.00 - ₹ 23,200.00 | |
 | D-(+)-Maltose monohydrate | Sigma Aldrich | ₹ 4,575.20 - ₹ 29,685.60 | |
 | Maltose monohydrate | Millipore | ₹ 9,100.00 - ₹ 30,160.00 | |
 | D-(+)-Maltose monohydrate | Sigma Aldrich | ₹ 17,848.60 | |
 | Maltose monohydrate | SAFC | ₹ 53,951.45 |
Description
- General description: Maltose monohydrate is commonly used as a tablet filler or excipient in pharmaceutical industry.[1]Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
- Application: Maltose Monohydrate may be used as a pharmaceutical reference standard for the determination of the analyte in drug mixtures by simulated moving bed chromatography.[2] It may also be used as a tablet filler with bisoprolol fumarate drug.[1]
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
- Other Notes: This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
- Footnote: To see an example of a Certificate of Analysis for this material enter LRAC2890 in the slot below. This is an example certificate only and may not be the lot that you receive.
SAFETY INFORMATION
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to USP 1375025
API family: maltose
CofA: current certificate can be downloaded
packaging: pkg of 1 g
technique(s): HPLC: suitablegas chromatography (GC): suitable
mp: 119-121 °C (dec.) (lit.)
application(s): pharmaceutical (small molecule)
format: neat
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to USP 1375025
API family:
maltose
CofA:
current certificate can be downloaded
packaging:
pkg of 1 g
technique(s):
HPLC: suitablegas chromatography (GC): suitable
mp:
119-121 °C (dec.) (lit.)
application(s):
pharmaceutical (small molecule)
format:
neat
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
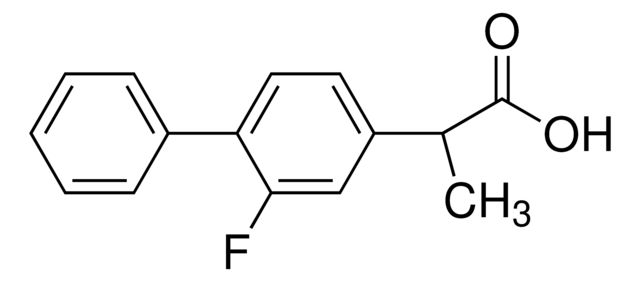
Agency: traceable to Ph. Eur. F0285200traceable to USP 1285750
API family: flurbiprofen
CofA: current certificate can be downloaded
packaging: pkg of 500 mg
technique(s): HPLC: suitablegas chromatography (GC): suitable
mp: 110-112 °C (lit.)
application(s): pharmaceutical (small molecule)
format: neat
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to Ph. Eur. F0285200traceable to USP 1285750
API family:
flurbiprofen
CofA:
current certificate can be downloaded
packaging:
pkg of 500 mg
technique(s):
HPLC: suitablegas chromatography (GC): suitable
mp:
110-112 °C (lit.)
application(s):
pharmaceutical (small molecule)
format:
neat
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
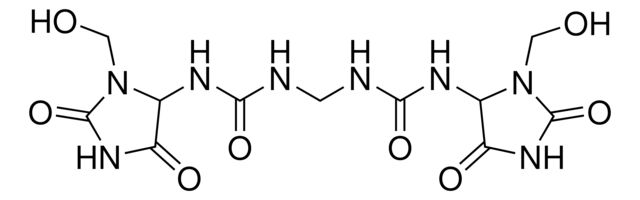
Agency: traceable to USP 1336806
API family: imidurea
CofA: current certificate can be downloaded
packaging: pkg of 1 g
technique(s): HPLC: suitablegas chromatography (GC): suitable
mp: __
application(s): pharmaceutical (small molecule)
format: neat
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to USP 1336806
API family:
imidurea
CofA:
current certificate can be downloaded
packaging:
pkg of 1 g
technique(s):
HPLC: suitablegas chromatography (GC): suitable
mp:
__
application(s):
pharmaceutical (small molecule)
format:
neat
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
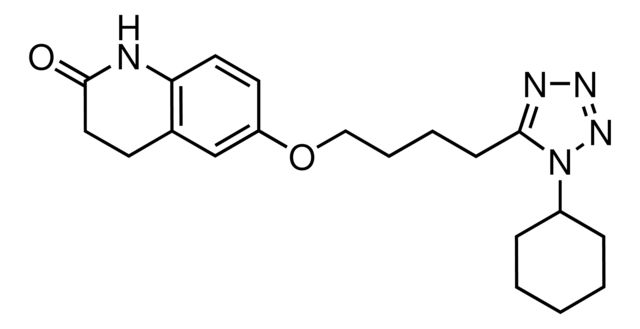
Agency: traceable to USP 1134153
API family: cilostazol
CofA: current certificate can be downloaded
packaging: package of 1 g
technique(s): HPLC: suitablegas chromatography (GC): suitable
mp: __
application(s): pharmaceutical (small molecule)
format: neat
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to USP 1134153
API family:
cilostazol
CofA:
current certificate can be downloaded
packaging:
package of 1 g
technique(s):
HPLC: suitablegas chromatography (GC): suitable
mp:
__
application(s):
pharmaceutical (small molecule)
format:
neat