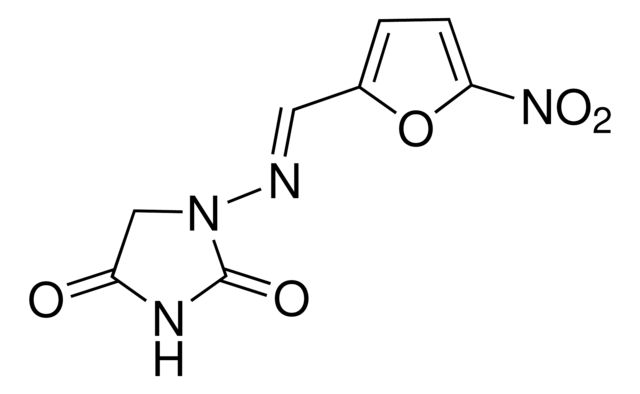
Nitrofurantoin
98.0-102.0% (EP, UV)
Manufacturer: Sigma Aldrich
CAS Number: 67-20-9
Synonym(S): N-(5-Nitro-2-furfurylidene)-1-aminohydantoin, Furadoxyl, Nitrofurantoine
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 10 G | N7878-10-G | In Stock | ₹ 4,070.20 |
| 25 G | N7878-25-G | In Stock | ₹ 4,167.63 |
| 100 G | N7878-100-G | In Stock | ₹ 14,722.00 |
N7878 - 10 G
In Stock
Quantity
1
Base Price: ₹ 4,070.20
GST (18%): ₹ 732.636
Total Price: ₹ 4,802.836
Quality Level
200
Assay
98.0-102.0% (EP, UV)
form
(Crystalline Powder or crystals)
color
yellow
solubility
DMF: soluble 50 mg/mL
antibiotic activity spectrum
Gram-negative bacteriaGram-positive bacteria
Mode of action
DNA synthesis | interferescell wall synthesis | interferesprotein synthesis | interferes
SMILES string
[O-][N+](=O)c1ccc(\C=N\N2CC(=O)NC2=O)o1
InChI
1S/C8H6N4O5/c13-6-4-11(8(14)10-6)9-3-5-1-2-7(17-5)12(15)16/h1-3H,4H2,(H,10,13,14)/b9-3+
InChI key
NXFQHRVNIOXGAQ-YCRREMRBSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
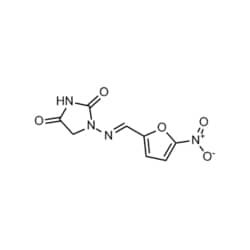 | Medchemexpress LLC HY-A0090 500mg Medchemexpress, Nitrofurantoin CAS:67-20-9 Purity:>98% | Medchemexpress LLC | ₹ 5,139.75 | |
 | Nitrofurantoin Pharmaceutical Secondary Standard, MilliporeSigma™ Supelco™ | MilliporeSigma Supelco | ₹ 11,303.00 | |
 | Sigma Aldrich Fine Chemicals Biosciences Nitrofurantoin 98.0-102.0% (EP, UV) | 67-20-9 | MFCD00003224 | 25G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 8,929.37 | |
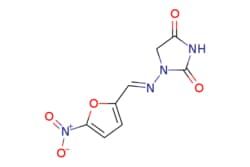 | eMolecules Ambeed / 1-(((5-Nitrofuran-2-yl)methylene)amino)imidazolidine-24-dione / 5g / 524999919 / A109585 / / 67-20-9 / MFCD00003224 / 238.159 / C8H6N4O5 | eMolecules | ₹ 2,878.26 | |
 | Nitrofurantoin | Supelco | ₹ 5,650.65 | |
 | Nitrofurantoin | Supelco | ₹ 10,207.98 | |
 | Nitrofurantoin | Sigma Aldrich | ₹ 40,756.13 | |
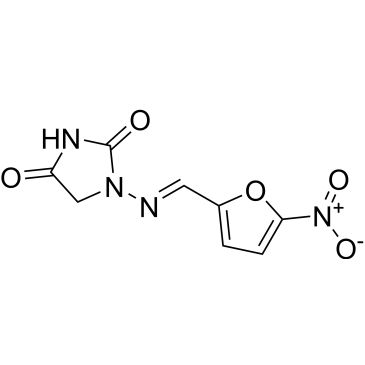 | Nitrofurantoin | ChemScene | ₹ 1,246.00 - ₹ 15,219.00 |
Description
- Application: Nitrofurantoin is a nitrofuran antibiotic that is used as a substrate of bacterial nitrofuran reductase to study the interactions of active metabolites with DNA, ribosomal proteins and metabolic and pro-oxidant processes. It is used to study nitrofurantoin-induced toxicity[1] and antibiotic resistance[2].Nitrofurantoin is suitable for killing L. monocytogenes-persisters in vitro.[3] Studies has described the use of antioxidants to mitigate the toxic effects of nitrofurantoin on human WI-38 fibroblasts in culture.[4] Alterations to the in vitro morphologic features, viability, and phagocytic activity of isolated bovine mammary polymorphonuclear leukocytes caused by various antibiotics, including nitrofurantoin, have been reported.[5]
- Biochem/physiol Actions: Nitrofurantoin is an antibactericidal compound that has been historically prepared by the reaction of 1-aminohydantoin sulfate and 5-nitro-2-furaldehyde diacetate. It shows activity against many Gram-positive and Gram-negative bacteria. Nitrofurantoin is effective against enterococci, staphylococci, streptococci, corneybacteria, many strains of Escherichia coli. Most strains of Proteus spp. and Pseudomonas aeurginosa are more resistant to this compound.[6] Nitrofurantoin is activated by bacterial flavoproteins (nitrofuran reductase) to actively reduce reactive intermediates that modulate and damage ribosomal proteins or other macromolecules, such as DNA. This inhibits DNA, RNA, protein, and cell wall synthesis which causes cell death[7]. Nitrofurantoin has a low resistance potential that is rapidly metabolized by mammals and is active against both Gram-positive and Gram-negative bacteria. It is also a pro-oxidant that is cytotoxic due to the generation of intracellular H 2O2. It is an inhibitor of glutathione reductase. Nitrofurantoin produces hepatotoxicity caused by the redox cycling of the nitro group and its radical anion which leads to oxidative stress[1].
- Other Notes: Keep container tightly closed in a dry and well-ventilated place.Light sensitive.
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Resp. Sens. 1 - Skin Sens. 1
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves